The Loophole with Delta-8
You may have seen storefront signs saying something like, “Delta-8 Sold Here”, and wondered what they could be selling. In states where recreational marijuana is not legal you would most likely think it certainly couldn’t be referring to that, and you would be right. It is technically not THC or delta-9-tetrahydrocannabinal, the main psychoactive ingredient in cannabis sativa. But according to the Agriculture Improvement Act of 2018, any part of the cannabis plant containing .3% or less of THC by dry weight is now defined as hemp. And there’s the loophole that has been exploited to permit delta-8 THC to be legally sold in markets that so far have restricted the legalization of marijuana.
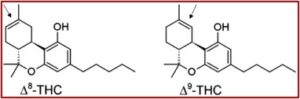
In December of 2018, the Senate easily passed the Agriculture Improvement Act of 2018, 87 to 13. In, “How Delta-8 THC Works, and Why Experts Are Worried About It,” The New York Times reported that at the insistence of Mitch McConnell, the bill legalized industrial hemp as a crop. The hope was that hemp could be used for construction products and plastic composites and help vendors of CBD, a non-psychoactive compound found in cannabis sativa or “hemp.” But what happened was the 2018 Farm Bill allowed hemp to be used as a precursor for synthesizing delta-8-THC, which has a nearly identical chemical structure to delta-9-THC. And delta-8 is psychoactive, producing a “high” similar to delta-9. See the graphic below from “Marijuana Variant of Concern.”
Here is why designating hemp as cannabis sativa containing less than .3% in the 2018 Farm Bill was so significant. According to the pro-marijuana website Weedmaps, most cannabis plants contain negligible amounts of CBD, less than 1%. However, hemp plants average between 12% and 18% CBD. The Center for Disease Control and Prevention (CDC) noted that CBD does not produce a high as THC does. At this point in time, there is only one FDA approved medicine containing purified CBD from hemp, Epidiolex, which is approved to treat seizure disorders.
But the 2018 Farm Bill removed “hemp from the federal Controlled Substances Act, effectively legalizing CBD if it comes from hemp.” CBD is now marketed in many consumer products: foods, oils, lotions, capsules, and cosmetics. Science is still learning about CBD and the CDC said CBD products are not risk free. They can lead to liver damage, drowsiness or sleepiness, diarrhea or changes in appetite, and mood changes like irritability. They may also lead to serious side effects when used in combination with other medicines or drugs.
There is a lot we do not know about CBD. Currently, we do not know how CBD use affects a person over time. We also do not know how different modes of CBD use (smoking, vaping, eating, applying to skin, etc.) affect a person.
CDC released a Health Alert Network (HAN) Health Advisory in 2021 to inform consumers that CBD can be synthetically converted into Delta-8 THC, which is psychoactive and not well understood. This alert warns consumers about the potential for adverse events due to insufficient labeling of products containing THC and CBD.
THC most often refers to delta-9 THC, the most common THC isomer in cannabis. But there are several other isomers that occur naturally in cannabis, including delta-8 THC, which is estimated to be approximately 50-75% as psychoactive as delta-9 THC. The CBD-to delta-8 THC conversion process uses a solvent, acid, and heat to produce concentrations of delta-8 THC higher than those found naturally in the cannabis plant. “This conversion process, used to produce some marketed products, may create harmful by-products that presently are not well-characterized.”
The 2018 Farm Bill led to an expanding and unregulated market for delta-8-THC, according to Leas et al, which sought to measure public interest in delta-8. The researchers looked at the global rate of recommended searches that mentioned delta-8-THC from January 2011 through August 2021. The search trends were stable from 2011 through 2019. But they increased by 257% from 2019 to 2020 and 705% from 2020 to August 2021. The global trend of delta-8-THC searches was driven primarily by increases in the U.S., where the rate increased by 466% from 2019 to 2020 and by 850% from 2020 to August 2021. “By 2021, the rate of searches for delta-8THC in the US was at least 10 times higher than [the] rate of delta-8-THC searches in any other country or territory.”
The growth in searches following legalization of hemp in the US as well as the greater interest in US States with more restrictive delta-9-THC policies suggests that delta-8-THC may be meeting a demand for legal use of THC in markets that do not permit use of delta-9-THC. The one-year lag following the legalization of hemp could potentially be explained by a need for developing an infrastructure to produce and ship delta-8-THC products. For example, one manufacturer claims to have created “USA’s first federally legal THC-dominant product since cannabis prohibition started,” after it developed a method of synthesizing delta-8-THC in September of 2019. By 2021, hundreds of Delta-8-THC manufacturers existed throughout the US, and many offered to ship products to consumers and wholesale to retailers in states that did not permit use of delta-9-THC.
While public interest in delta-8-THC seemed to be concentrated in the US, some manufacturers have opened offices in Europe. One manufacturer, Just Delta, has offices in the UK. Leas et al said global and US jurisdictions should clarify whether methods of converting cannabinoids to THC compounds are legal under existing hemp and cannabis laws. They recommended a public-health-focused approach that clarifies definitions of THC compounds to include delta-8-THC and other THC isomers; and disallows the use of methods that convert CBD to THC, “at least until these can be determined to be safe.”
The NYT article, “How Delta-8 THC Works,” reported that a survey of delta-8 users said they were less anxious, less paranoid, and had a nicer high than with delta-9 THC. “The most common experiences when using delta-8 were relaxation, euphoria and pain relief.” There were reports of some difficulty concentrating, problems with short-term memory and an altered sense of time, but not to the same extent as with regular marijuana. The explanation for the differences between delta-8 THC and delta-9 THC is probably that there’s less delta-8 THC in the CB1 receptors, “so people are less likely to experience the more distressing symptoms” when they get too high.
Manufacturers of delta-8 products argue that delta-8 may chemically be THC, but legally it now is hemp. “Since you can extract CBD from hemp, and CBD is not THC, [then] it’s still considered hemp.” The lack of regulation around delta-8 in the US is the biggest concern of many public health experts. In a paper published in December of 2022, none of the delta-8 products tested contained the amount of delta-8 they claimed. All 27 had potentially harmful byproducts, presumably from the manufacturing process, including lead and mercury.
Between January 2021 and February 2022 national poison control centers handled over 2,000 calls about delta-8. Forty-one percent involved children accidentally ingesting products with delta-8. “One of those cases resulted in death.” Without federal regulation, 14 states have banned delta-8, or all unregulated forms of THC, including delta-10. Ironically, this includes several states where recreational marijuana is legal, including Colorado and New York. Delta-10 is illegal in Colorado and New York as well.
Eric Leas, the lead researcher in the above study that assessed public interest in delta-8-THC, said in “The Hemp Loophole,” that “the loopholes that allow THC compounds to be sold as hemp ought to be closed.” He said the regulatory system for recreational marijuana makes it a safer product than delta-8. The manufacturing quality checks and other regulatory requirements such as labeling rules about potency and licensing distributers act as important public health standards. None of these protections currently exist for delta-8. All the experts interviewed for “How Delta-8 THC Works,” including those supportive of legalizing marijuana, recommended against using delta-8. “There is no way to ensure its safety.”