Don’t Legalize Psychedelics Until the Guardrails Are In Place

Starting in the Summer of 2023, there was a flurry of announcements about psychedelics being used to treat psychiatric disorders. Australia became the first country to permit psychiatrists to prescribe psychedelics for depression or post-traumatic stress disorder (PTSD). MAPS formally submitted a New Drug Application to the FDA for the approval of MDMA-assisted therapy for PTSD. California passed Senate Bill 58, which would decriminalize psychedelics and make several naturally occurring psychedelic substances like psilocybin, and mescaline legal within the state. But then, Governor Newsom vetoed the bill, saying more needed to be done before decriminalizing hallucinogens.
The legislation would have required the California Health and Human Services Agency to make recommendations to lawmakers on the therapeutic use of psychedelics after their decriminalization. Newsom vetoed Senate Bill 58 because there were no existing regulated treatment guidelines; no dosing information or therapeutic guidelines. There wasn’t a medical clearance attesting there were no underlying psychoses; or rules against exploitation during guided treatments. He said: “Unfortunately, this bill would decriminalize possession prior to these guidelines going into place, and I cannot sign it.”
The Associated Press noted how public opinion has shifted to support the therapeutic use of psychedelics. Psilocybin was designated as a “breakthrough therapy” for depression in 2019 and the FDA published guidelines on clinical trials with psychedelic drugs. MAPS anticipates an FDA decision on MDMA-assisted therapy very soon. The FDA acknowledged this growing interest in the therapeutic potential of psychedelic drugs and noted a need for applicants to attend to various conditions in order to facilitate its review process of psychedelics. “Designing clinical studies to evaluate the safety and effectiveness of these compounds presents a number of unique challenges that require careful consideration.”
The document describes basic considerations throughout the drug development process including trial conduct, data collection, subject safety and new drug application requirements. For example, psychedelic drugs may produce psychoactive effects such as mood and cognitive changes, as well as hallucinations. As a result, there is the potential for abuse of these drugs, which is a drug safety issue that requires careful consideration and putting sufficient safety measures in place for preventing misuse throughout clinical development. For psychedelics that are currently Schedule I controlled substances, the draft guidance notes that activities associated with investigations under an Investigational New Drug Application must comply with applicable Drug Enforcement Administration regulatory requirements.
The California Coalition for Psychedelic Safety and Education, which opposed Senate Bill 58 thought more safeguards were necessary before decriminalization of psychedelics in California. “We’re grateful that Governor Newsom listened to some of the top medical experts, psychedelic researchers and psychiatrists in the country who all warned that legalization without guardrails was at best premature for both personal and therapeutic use.” They thought there should be public education campaigns, safety protocols and emergency response procedures “to keep Californians safe.”
The Australian announcement noted above surprised some scientists in that country when it was made. The Australian & New Zealand Journal of Psychiatry published an editorial by psychiatrist Steve Kisley, “The down-scheduling of MDMA and psilocyin(e): Too fast and too soon.” Australia’s Therapeutic Goods Administration (TGA) had rejected a petition to down-schedule both substances as recently as 2020. As a result of criticism, the TGA commissioned an independent report into the risks and benefits of MDMA and psilocybin.
The report concluded while the substances showed promise in highly selected populations in closely supervised settings, the trial quality was variable “with only small proportions of potential participants included in randomised comparisons.” The certainty of evidence was rated low or very low using the Cochrane Collaboration’s GRADE framework. The TGA confirmed its decision, but inexplicably approved the down-scheduling of MDMA for PTSD and psilocybin for treatment-resistant depression. Dr. Kisley said:
In the view of this author of the independent report to the TGA, the decision to down-schedule is ahead of the available scientific evidence especially as unresolved issues remain surrounding relapse, long-term safety and the challenges of conducting randomised controlled trials in this area.
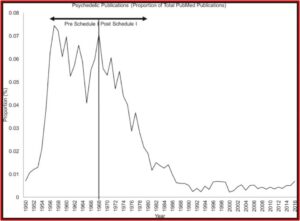
The wisdom of a slower, more cautious approach to decriminalizing psychedelics or approving their use to treat mental health disorders was illustrated in the May 2024 edition of the journal Addiction, where a study of hallucinogen-associated emergency department visits and hospitalizations in California from 2016 to 2022 showed increases by 54 and 55% respectively. The researchers thought this was a concerning trend, especially given that public opinion is that hallucinogens are low-risk. “There is a pressing need for broader and more rigorous tracking of the harms associated with these emerging substances on the individual and population level, especially as policy changes increase access.”
Science Alert described a recent study done at Imperial College London that highlighted the potential risks of psychedelic use and therapy for individuals with personality disorders. The study collected self-reported data from 807 people who used psychedelics in various settings, including recreational and therapeutic use. The Warwick-Edinburgh mental wellbeing scale was given before and after participants used psychedelics. Only 16% of all participants reported an overall negative response. “But a significant portion of these negative experiences (31%) [5 cases of 16] were reported by people with a history of personality disorders.”
There were several limitations to the study, including the reliance on self-reported data, a 56% dropout rate, and the small number of participants with a history of personality disorders. There were also variations in the types and doses of psychedelics used. The study’s method of participant selection may also lead to biased results. Yet, the potential benefits of psychedelics for mental health were acknowledged, “stressing the need for careful screening for personality disorders.”
Using psychedelics safely and effectively requires a personalised approach. This is especially true for vulnerable people. This highlights the importance of refining psychedelic therapy to make it safe and effective for all.
As we explore the expanding territory of psychedelic therapy, it’s vital to understand how these substances interact with mental health conditions – including personality disorders.
In a study published May 8, 2024 in Nature, Warren et al highlighted how psychedelics interacting with serotonin receptors could produce potential therapeutic benefits with depression and anxiety. In “Psychedelics Activate Serotonin to Produce Antidepressant Effect,” Audrey Warren said: “Our study highlights, for the first time, how serotonin receptors like 5-HT1A likely modulate the subjective effects of the psychedelic experience and also play a potentially pivotal role in their clinically observed therapeutic outcome.” The researchers believed their study will lead to a better understanding of the pharmacology of psychedelics involved with many receptor types. “We’ve demonstrated that psychedelics have complex physiological effects that span many different receptor types … and are now ready to build on that finding to develop improved therapeutics for a range of mental health disorders.”
But before this happens, rigorous, replicated studies of the therapeutic use of psychedelics should occur before they are decriminalized or made available to treat mental health disorders. A medical clearance procedure that screens for underlying psychoses needs to be put in place. Dosing information and regulated treatment guidelines needs to be established. In other words, don’t legalize psychedelics until the guardrails are in place to ensure the safe and effective use of these compounds.