In case you missed it, Janssen announced the FDA’s approval of a new indication for Spravato “to treat depressive symptoms in adults with major depressive disorder with suicidal ideation of behavior.” Spravato (esketamine) was originally approved by the FDA as a fast-acting antidepressant for treatment-resistant depression in March of 2019. Janssen and the FDA seem to be forging ahead despite misgivings expressed with the approval of esketamine from a number of sources, including members of the advisory board that approved Spravato. But perhaps the most damning and confusing endorsement of Spravato can be found in Janssen’s announcement of this new indication.
The announcement was released on August 3, 2020 and stated its approval was based on Phase 3 data showing that Spravato reduced depressive symptoms in as little as four hours in some patients, with symptom improvement maintained through the four-week treatment period. “Spravato is the first and only approved medicine that has been shown to reduce depressive symptoms within 24 hours.” First Spravato was approved as a fast-acting antidepressant for treatment resistant depression, and then it was approved to treat depressive symptoms with a new population: adults with major depression and suicidal ideation behavior. But you would be wrong to conclude the new approval was because it was shown to treat suicidal ideation in the new population. Here is what the August 3rd Janssen announcement said:
The effectiveness of Spravato in preventing suicide or in reducing suicidal ideation or behavior has not been demonstrated. Use of Spravato does not preclude the need for hospitalization if clinically warranted, even if patients experience improvement after an initial dose of Spravato. Spravato carries a Boxed Warning regarding a Risk Evaluation and Mitigation Strategy (REMS) and the risk of suicidal thoughts and behaviors.
So Janssen was proud to announce Spravato was approved by the FDA to treat depressive symptoms other than suicidal ideation in adults with major depression and suicidal ideation! The doublespeak does not end there. The paragraph from which the above quote was taken referred its readers to “Important Safety Information” below in the press release. In the section on “Important Safety Information,” the press release repeated the two conditions for which the FDA approved Spravato: adults with treatment-resistant depression and depressive symptoms in adults with major depressive disorder with suicidal thoughts or actions. It also warned that antidepressant medicines “may increase suicidal thoughts and actions in some people 24 years of age and younger.” And then you see a similar disclaimer to the one above:
It is not known if Spravato is safe and effective for use in preventing suicide or in reducing suicidal thoughts or actions. Spravato is not for use in place of hospitalization if your healthcare provider determines that hospitalization is needed, even if improvement is experienced after the first dose of Spravato.
Spravato was approved “to treat depressive symptoms in adults with major depressive disorder with suicidal ideation of behavior,” but not to treat the symptom of “recurrent suicidal ideation”! See Medscape for “What are the DSM-5 criteria for diagnosis of major depressive disorder?” The same doublespeak is included in advertisements for Spravato. Erick Turner, a former reviewer of psych meds for the FDA, posted the following advertisement on Twitter:
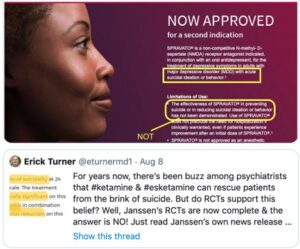 Note his highlights of the same information discussed above. In an earlier post, dated August 6, he said: “The FDA has *not* approved esketamine for suicidal ideation. But the indication for which it was approved was worded [in a way that] will mislead many people into thinking that it was. How could the FDA allow Janssen to get away with this slick marketing?” In case you are skeptical about the above advertisement being photoshopped, here is a link to a similar ad for Spravato, that uses the same language highlighted above.
Note his highlights of the same information discussed above. In an earlier post, dated August 6, he said: “The FDA has *not* approved esketamine for suicidal ideation. But the indication for which it was approved was worded [in a way that] will mislead many people into thinking that it was. How could the FDA allow Janssen to get away with this slick marketing?” In case you are skeptical about the above advertisement being photoshopped, here is a link to a similar ad for Spravato, that uses the same language highlighted above.
This seems to be some marketing spin placed on the results of phase 3 clinical trials for esketamine. ASPIRE I and ASPIRE II were said to suggest that esketamine nasal spray may address the unmet need for a rapid acting antidepressant in patients with MDD at imminent risk of suicide. Depressive symptoms were significantly decreased in both trials. However, while there was improvement in the severity of measured suicidality, there were no statistically significant differences in the severity of suicidality. ASPIRE I, which was published in the Journal of Clinical Psychiatry, also said the study site investigator did not think any suicide-related adverse events were related to esketamine. The trials were with patients with major depressive disorder (MDD) who were at high risk of suicide, but let’s not simply accept the site investigator’s assessment and dismiss the significance of the suicide-related adverse events.
There were 4 attempted suicides and 1 completed suicide among patients with esketamine treatment. In ASPIRE II, there were 3 attempted suicides among patients with esketamine treatment. Both ASPIRE I and ASPIRE II pointed out that standard antidepressants effectively treat depressive symptoms, including suicidal ideation, but they require 4-6 weeks to have a full effect. Although there were significantly decreased symptoms of depression, there were still suicide attempts and one completed suicide in ASPIRE I and II, despite the rapid treatment effect with esketamine. So esketamine can’t claim that it provides rapid relief for suicidal ideation within the population its was just approved to treat—adults with major depressive disorder with suicidal ideation of behavior.
Joanna Moncrieff and Mark Horowitz published the following comments on esketamine in the British Journal of Medicine on October 8, 2019. There had been a BMJ editorial endorsing esketamine, “Esketamine for treatment resistant depression.” They said they were surprised to see the endorsement, as esketamine has been licensed on flimsy evidence for treatment resistant depression. “The very existence of Treatment Resistant Depression is a testimony to the ineffectiveness of the pharmacological approach to depression.”
Moncrieff and Horowitz pointed out the esketamine trials were only for 28 days, and noted there is almost no data on the adverse effects from long-term treatment. Serious adverse effects from long-term treatment will take time to come to light. “Yet we know from the recreational drug scene that ketamine use is associated with severe bladder problems and that prolonged use of euphoriant drugs like ecstasy can cause depression in itself.” This means that crucial research data was left to be gathered after the drug was licensed in the US. Doing so “puts the public at risk and sets depressed patients up as unwitting guinea pigs in a huge and unregulated pharmaceutical experiment.”
A report of post-marketing adverse events with esketamine was published in the journal Psychotherapy and Psychosomatics in August 2020. In “Post-Marketing Safety Concerns with Esketamine,” Gastaldon et al reported their analysis of data found in the FDA Adverse Event Reporting System (FAERS). The researchers looked at esketamine-related adverse events between its original approval by the FDA in March of 2019 and March of 2020. The FAERS database contained 962 cases of esketamine-related adverse events such as: dissociation (212), sedation (173), feeling drunk (20), anxiety (63), suicidal ideation (64), depression (65), depressed mood (12) and completed suicide (11). Note again where suicidal ideation and completed suicide still occurred. The link here is to the abstract of the article, but you can access “Free Supplementary Material” that contains the number of cases I’ve included here in parentheses.
There have been concerns expressed about Spravato/esketamine from the time it was initially approved by the FDA. The independent advisory committee voted 14-2 that the benefits of esketamine outweighed the risks. But STAT wrote some experts weren’t convinced there was enough data at the time to show that esketamine was effective. They wanted data on how the drug should be used in the long run.
Johnson & Johnson, the parent company to Janssen, submitted five Phase 3 studies: three short-term studies, one maintenance study, and a long-term safety study. Two of those were positive. One of the studies was of adults under 65 with treatment-resistant depression. The second was a maintenance-of-effect study, where participants who responded to esketamine in one of the short-term studies were then randomly assigned to either taking esketamine or a placebo. Historically, such withdrawal studies have not been counted toward the needed two studies.
At the time, Erick Turner was a member of the FDA advisory committee that evaluated esketamine. When the panel met to vote on recommending approval to the FDA, Turner couldn’t participate because of travel reasons. He said: “The threshold has been two adequate and well-controlled trials. In this case, they only got one … Based on that, I would have voted no.” Julie Zito was one of the two advisory committee members who did vote “no” on whether the benefits of esketamine outweighed its risks. She said if Spravato was approved, she hoped providers, patients and the families of patients would work together to keep tabs on possible side effects and how the drug was working.
Remember that Gastaldon et al reported esketamine gathered together 962 adverse event cases on FAERS in its first year on the market. FAERS reported that data as of June 30, 2020, reported 1,305 adverse events; 619 serious cases that included 39 deaths, 18 of which were completed suicides. The case count for dissociation had risen to 266; to 221 for sedation; to 83 for suicidal ideation; and to 84 for depression. There were another 343 adverse events from esketamine reported to FAERS in three months; and 7 more completed suicides.
Even though it has been given a Risk Evaluation Mitigation Strategy (REMS), long-term adverse events will likely include concerns with substance misuse. Like ketamine, esketamine ‘s chemical cousin, it is a controlled substance (Schedule III). Ketamine is known recreationally as the popular club drug “Special K.”
The data used to approve Spravato for treatment resistant depression was flimsy to begin with. Now the FDA has approved it to treat adults with major depressive disorder who struggle with suicidal ideation. This widens the population approved to use Spravato and increases the market to whom Janssen can legally market Spravato. Nothing good will come of this. The “good news” in Janssen advertising is now Spravato can be marketed to adults with major depressive disorder and suicidal ideation. But ironically, Spravato has not been demonstrated to be effective in preventing suicide or in reducing suicidal ideation.
This kind of rhetoric reminds me of George Orwell’s classic book 1984, in which he wrote: “Doublethink means the power of holding two contradictory beliefs in one’s mind simultaneously, and accepting both of them.” Does it seem that Janssen is attempting to get some people to doublethink with Spravato? For more on Spravato/esketamine, see “Hype And Concern With Esketamine” and “Red Flags With Spravato.”

