The Business of Legalizing Marijuana in PA

In the midst of our national political news, there was an announcement by John Fetterman, the Lieutenant Governor of Pennsylvania, that he is formally exploring a run for the U.S. Senate in 2022. According to Marijuana Moment, legalizing weed is a central part of his political platform. Fetterman said: “We’re going to get there. There’s never going to be a time when that’s not true. It’s inevitable. They know it, I know it, everyone knows it.” He even placed a marijuana flag on the balcony of his office in the Pennsylvania Capitol building as lawmakers were sworn in ahead of the 2021 legislative session.
If he decides to run, this will be his second try for the U.S. Senate. In 2016 Fetterman lost in the Democratic primary, but earned 20 percent of the vote in a four-way race. Two years later, he ran for Pennsylvania Lieutenant Governor and won. Weeks after taking office, he began a listening tour through all 67 counties of the state “to engage with Pennsylvanians about legalizing marijuana.” He said: “We want full legalization. I mean, that’s really the net goal.”
Given Fetterman’s commitment to full legalization, which means legalizing marijuana for recreational use, Pennsylvanians should know more about the potential consequences of this proposed marijuana legalization. If HB 2050 is passed, adults 21 and over could possess any amount of cannabis. Public consumption is prohibited. Adults 21 and older could cultivate 50 square feet of mature, flowering cannabis plants within a private residence. And adults could gift up to one ounce from cannabis grown in their private residences.
Going “full Colorado,” as Fetterman has called it has had negative consequences in Colorado, with the crime rate increasing 11 times faster than the rest of the nation since legalization, according to the Colorado Department of Public Safety. SAM, Smart Approaches to Marijuana, reported the number of drivers intoxicated with marijuana and involved in fatal accidents increased 88% from 2013 to 2015. The proportion of Colorado youth who reported marijuana use in the past 30 days was higher than the national average. Fuller discussion of these facts and more can be found in, “Should Pennsylvania Go ‘Full Colorado’ With Marijuana?” Part 1 and Part 2. Also see, “From The Frying Pan Into the Fire With Recreational Marijuana in PA.”
There are also concerns with the potential consequences with high-potency marijuana. Today’s cannabis is much more powerful than it was in the past. The U.S. Surgeon General published an advisory on Marijuana Use and the Developing Brain. Citing a study published in Biological Psychiatry, “Changes in Cannabis Potency over the Last Two Decades,” he said the THC concentration in commonly cultivated marijuana plants increased three-fold from 1995 through 2014, 4% to 12%. Researchers of a different study of legal cannabis sold in Washington State dispensaries found that the median THC levels varied from 17.7% to 23.2%.
Concentrated THC products, known as “dabs,” “wax,” “budder,” are becoming more widely available to recreational users and were found in yet another study, “To Dab or Not to Dab,” to range from 23.7% to 75.9% THC. The authors also cited published case reports that showed significant psychosis, neurotoxicity and cardiotoxicity with dabs. They described three males in their teens or twenties who used some form of dab. Two of the subjects had paranoia-like symptoms and one subject had seizure-like activity. Analysis of the dab sample used by one of the subjects was 20.5% THC without any detectable level of cannabidiol (CBD). The Surgeon General said the risks of physical dependence and other negative consequences increase with exposure to high concentrations of THC. “Higher doses of THC are more likely to produce anxiety, agitation, paranoia, and psychosis.”
A systematic review and meta-analysis published in the Lancet found that acute administration of THC induced increased positive (psychotic), negative (i.e., poor rapport) and general (depression) psychiatric symptoms. “These findings demonstrate that the acute administration of THC induces positive, negative, and general psychiatric symptoms with large effect sizes.” However, CBD did not induce psychiatric symptoms and there was inconclusive evidence that it moderated the initiation of psychiatric symptoms by THC.
These findings highlight the acute risks of cannabis use, which are highly relevant as medical, societal, and political interest in cannabinoids continues to grow.
In addition to mental health concerns with the legalization of marijuana, there are physical health concerns. Marijuana smoke contains some of the same toxic combustion products in tobacco smoke, which raises the possibility that exposure to some smoke-related toxicants could have adverse effects on the health of heavy cannabis users.
In another Lancet study, the plasma and urine levels of exclusive marijuana smokers, exclusive tobacco smokers, dual users of both substances and non-smokers were compared. Participants were classified as marijuana or tobacco smokers based upon self-report and detection of nicotine or THC metabolites in their plasma or urine. Marijuana smoking was independently associated with smoke-related toxicants including acrylamide and acrylonitrile metabolites, which are known to be toxic at high levels. But levels were lower when compared with those associated with tobacco smoking. Researchers also found elevated levels of an acrolein metabolite that may identify adults at risk of cardiovascular disease. The senior author of the study told Science Tech Daily:
Marijuana use is on the rise in the United States with a growing number of states legalizing it for medical and nonmedical purposes — including five additional states in the 2020 election. The increase has renewed concerns about the potential health effects of marijuana smoke, which is known to contain some of the same toxic combustion products found in tobacco smoke.
While there is less of a risk of cardiovascular disease when smoking marijuana than tobacco, there is a clear risk of experiencing psychiatric symptoms of psychosis and depression. This occurs as a result of the psychoactive substance in the cannabis plant, THC. However, another of the 66 chemicals (cannabinoids) in cannabis, cannabidiol (CBD), does not induce psychiatric symptoms. This distinction is crucial when considering whether or not to broaden the existing use of medical marijuana in Pennsylvania to include recreational use.
The FDA has approved only one CBD product Epidiolex, a prescription drug to treat two rare, severe forms of epilepsy. It is also illegal to market CBD by adding it to a food or drink; or currently to label it as a dietary supplement. According to Marijuana Moment, the FDA is in the process of developing regulations for hemp-derived cannabidiol products and is actively exploring ways to permit lawful sales of CBD as a dietary supplement. In 2019 the World Health Organization (WHO) said hemp-derived CBD did not contain more than .2 percent THC and was not under international control.
In a 2017 report to the WHO, the Expert Committee on Drug Dependence said in humans, “CBD exhibit no effects indicative of any abuse or dependence potential.” It is generally well tolerated and has a good safety profile. Reported adverse effects were thought to be a result of interactions between CBD and patients’ existing medications. “CBD has been demonstrated as an effective treatment of epilepsy in several clinical trials. . . There is also preliminary evidence that CBD may be a useful treatment for a number of other medical conditions.”
The evidence that CBD may be a useful treatment for a number of other medical conditions is not as scientifically sound as the research for treating epilepsy. For most indications, there is only pre-clinical evidence. Consistent with its properties, the range of conditions for which CBD has been assessed is diverse. Why can’t there be changes in how CBD from cannabis is scheduled and regulated to facilitate further research into these preliminary findings? The following table in the WHO report represents a review of the various therapeutic applications for CBD found in “Cannabidiol: State of the art and new challenges for therapeutic applications.”
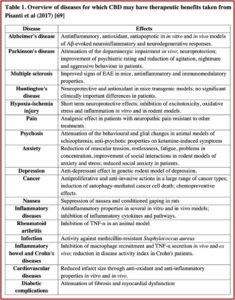 The WHO commented that since CBD was not considered a drug of abuse (as was THC) and at the same time was legal and not regulated, a market for CBD-based products for medical purposes (such as CBD oil, tinctures and vapors) has rapidly expanded. It flourishes “in a no man’s land with potential health dangers for patients and all end-users.” The WHO cautioned that the lack of regulation with these products cannot guarantee the patient “the quality of the product itself, the effective dosage of CBD that is fundamental for its therapeutic effectiveness, the purity and the absence of chemical or microbiological contaminations, thus raising critical public safety concerns.”
The WHO commented that since CBD was not considered a drug of abuse (as was THC) and at the same time was legal and not regulated, a market for CBD-based products for medical purposes (such as CBD oil, tinctures and vapors) has rapidly expanded. It flourishes “in a no man’s land with potential health dangers for patients and all end-users.” The WHO cautioned that the lack of regulation with these products cannot guarantee the patient “the quality of the product itself, the effective dosage of CBD that is fundamental for its therapeutic effectiveness, the purity and the absence of chemical or microbiological contaminations, thus raising critical public safety concerns.”
Regulatory changes with regard to CBD are needed in the U.S. At the end of December 2020, the FDA announced it had issued five more warning letters to companies selling CBD products in ways that violated the Federal Food, Drug and Cosmetic Act (FD&C Act). “All five warning letters address the illegal marketing of unapproved CBD products claiming to treat medical conditions. In addition, they address violations relating to the addition of CBD to food, and the impermissible marketing of CBD products as dietary supplements.” Under the FD&C Act, any product intended to diagnose, cure, treat or prevent a disease and any product intended to affect the structure or function of the body of humans or animals, is considered to be a drug.
In “FDA is Committed to Sound, Science-based Policy on CBD,” the FDA said the Agriculture and Improvement Act of 2018, the Farm Bill, removed cannabis and cannabis derivatives that are very low in THC from the definition of marijuana in the Controlled Substances Act while specifically preserving the FDA’s responsibility over such products. If a product is being marketed as a drug, meaning it is intended to have a therapeutic effect as in treating a disease, then it is regulated as a drug. Over the past several years the FDA has issued several warning letters to companies for marketing unapproved new drugs claiming to contain CBD, including for uses such as treating cancer or Alzheimer’s disease. “These products were not approved by the FDA for the diagnosis, cure, mitigation, treatment, or prevention of any disease.”
Legislative action is needed, but not to legalize recreational marijuana. An official distinction between THC and CBD should be made that permits research into the therapeutic potential of CBD, while keeping THC as a controlled substance at a less restrictive Schedule. Marijuana was listed as a Schedule I Controlled Substance in 1970. This has hampered the scientific investigation of the therapeutic benefits of CBD as well as the potential harms (or lack thereof) from THC to be done.
There is enough evidence of the therapeutic potential of CBD to research the above-noted therapeutic benefits and we desperately need legislation that permits this. There is also evidence that high levels of THC can be a catalyst to the emergence of psychotic symptoms in some users—and we need to protect those users from this danger. The science is clear enough to stop the push towards legalizing recreational marijuana in Pennsylvania while we permit the scientists to do their research into the potential benefits of CBD and the adverse effects of THC. Activists and those in favor of legalizing recreational marijuana like Lieutenant Governor Fetterman are really seeking to legalize THC, a psychoactive chemical in cannabis with serious adverse effects and significant profit potential for some. We need to clearly see this.