
Alkermes seems to be presenting contradictory opinions when it comes to promoting two of its drugs. When discussing Vivitrol, its extended release injectable version of naltrexone, Alkermes refers to it as “nonaddictive.” This is in contrast to other FDA-approved addiction medications such as methadone or buprenorphine, which are opioids. Alkermes has also persisted in its attempts to get ALKS 5461 approved by the FDA as an adjunctive medication to treat major depression. The active ingredient in ALKS 5461 is buprenorphine and Alkermes has emphasized that upon discontinuation of ALKS 5461 there was no evidence of withdrawal.
The conundrum is rhetorically-based and not scientific. Buprenorphine is classified as a Schedule III controlled substance, meaning it has “a moderate to low potential for physical and psychological dependence.” Yet when ALKS 5461 is being promoted, research findings conclude “There was no evidence of withdrawal upon discontinuation of treatment with ALKS 5461.” Michael Thase, the Alkermes poster presenter for ALKS 5461 at the 2018 American Psychiatric Association annual conference was quoted by Healio as saying, “there was essentially no hint whatsoever of opiate-like withdrawal or discontinuation symptoms.”
But when buprenorphine is a medication-assisted treatment (MAT) in competition with Vivitrol, it is described by Alkermes and its lobbyists as being addictive in contrast to naltrexone, the ingredient in Vivitrol, which is not addictive. STAT News published an article written by Daniel Wolf, the director of international harm reduction development at the Open Society Foundation. Wolf cited and linked investigations by the New York Times, National Public Radio and ProPublica noting how Alkermes promoted Vivitrol at the expense of other MAT drugs like buprenorphine. One example, found in the NPR article, was where the Indiana Senate passed a bill approving the use of “a federal Food and Drug Administration-approved long acting, nonaddictive medication for the treatment of opioid or alcohol dependence.” The only drug approved by the FDA that meets that description is Vivitrol.
An investigation by NPR and Side Effects Public Media has found that in statehouses across the country, and in Congress, Alkermes is pushing Vivitrol while contributing to misconceptions and stigma about other medications used to treat opioid addiction.
Wolfe noted for STAT News that the investigations he cited showed how Alkermes marketers and lobbyists derided the daily administration of methadone and buprenorphine. The pitch is apparently working, as the sales of Vivitrol rose 600% between 2011 and 2017. Legislators in 15 states have written Vivitrol (by brand name) into their laws. And multiple jurisdictions now require drug offenders to agree to use the medication if they want to avoid imprisonment. In effect, state established Drug Courts have become “Vivitrol courts.”
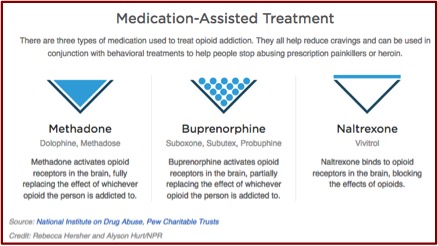
Alkermes highlighted Vivitrol’s properties as an opioid antagonist. This means it blocks (instead of activating) the effects of opioids like methadone, burpernorphine and heroin on opioid receptors in the brain. Naltrexone, the active ingredient in Vivitrol, will not get you high. Methadone and buprenorphine can be used alone or in conjunction with other drugs to produce a high. Their differences as MAT treatment are illustrated in the following graphic, which was found in the NPR article.
 Turning to ALKS 5461, we see that Alkermes had a series of setbacks as it attempts to have the FDA approve ALKS 5461 as an adjunct treatment for major depression. As FierceBiotech noted, Alkermes failed to meet their primary endpoints in two of three phase 3 clinical trials for ALKS 5461. “But Alkermes used the success of the third trial to paint the overall dataset as supportive of the efficacy of ALKS 5461.” What Alkermes did is use its statistical analysis of the second failed clinical trial (FORWARD-4) to discover a significant result within a subpopulation of the study that wasn’t targeted by its primary endpoint. They then modified their methodology and analysis of the third clinical trial (FORWARD-5) to match the post-hoc analysis and it successfully showed a statistical significance.
Turning to ALKS 5461, we see that Alkermes had a series of setbacks as it attempts to have the FDA approve ALKS 5461 as an adjunct treatment for major depression. As FierceBiotech noted, Alkermes failed to meet their primary endpoints in two of three phase 3 clinical trials for ALKS 5461. “But Alkermes used the success of the third trial to paint the overall dataset as supportive of the efficacy of ALKS 5461.” What Alkermes did is use its statistical analysis of the second failed clinical trial (FORWARD-4) to discover a significant result within a subpopulation of the study that wasn’t targeted by its primary endpoint. They then modified their methodology and analysis of the third clinical trial (FORWARD-5) to match the post-hoc analysis and it successfully showed a statistical significance.
In previous articles, “Nearsighted Drug Development” and “The ‘Hotel California’ Effect,” I said this seemed like cheating, even though it was allowed. Alkermes then wanted the FDA to count its failed FORWARD-4 trial as a successful one, based on its post hoc statistical analysis. So far the FDA hasn’t bought the Alkermes attempts to justify its claims. In late January of 2018 Alkermes submitted a New Drug Application to the FDA for ALKS 5461. The company claimed ALKS 5461 “demonstrated a consistent profile of antidepressant activity, safety and tolerability in the adjunctive treatment of MDD.”
Then on April 2, 2018, the FDA informed Alkermes it was refusing to review the NDA application for ALKS 5461, saying there was “insufficient evidence of overall effectiveness for the proposed indication.” They wanted to see “additional well-controlled clinical trials” prior to the resubmission of ALKS 5461, disagreeing with the Alkermes attempt touse the success of the third trial to the overall dataset as supportive of ALKS 5461 efficacy. The FDA also wanted a bioavailability study to generate more bridging data between ALKS 5461 and buprenorphine. Alkermes disagreed with the FDA’s conclusions and planned to request a Type A meeting where they could clarify what additional information the FDA needs. FierceBiotech said:
The FDA’s refusal to buy into that line of thinking is a blow to people in Alkermes and beyond. Having talked up the strength of the dataset, Alkermes CEO Richard Pops and his colleagues emerge from the setback with dented reputations. More broadly, the FDA’s refusal to even review the data is a blow for any biotech hoping the arrival of Scott Gottlieb, M.D., as commissioner would usher in an era in which the FDA waves through drugs with patchy data packages.
Two weeks later, the FDA rescinded its refuse-to-file letter. FierceBiotech said: “The company did not provide the FDA with additional data or analyses and the agency expects to return a decision by Jan. 31, 2019.” Alkermes CEO Richard Pops said the FDA’s initial refusal was based on a “misunderstanding” of the NDA submission. He declined to say what the FDA initially thought was missing from the NDA. “I think it just took a while to get that lens focused the right way for FDA to accept the file.”
While ALKS 5461 is back on schedule, the FDA’s about-face is by no means a guarantee of success—the agency has deemed the data complete enough for review, but could still reject it.
At the May 2018 American Psychiatric Association Annual Meeting, Alkermes presented a poster on its research into the “Long-Term Efficacy, Safety and Tolerability of Adjunctive ALKS 5461 in Patients With Major Depressive Disorder.” The reported results of a completed long term study (NCT02141399) were that 49% of the 1454 enrolled patients completed the 1-year study, 11% discontinued due to an adverse event. The remission rate, defined as a score of 10 or less on the MADRS-10, was 52.5%. Time to remission was 59.0 days. Adverse events occurring with a frequency of less than 10% were nausea, headache, constipation, dizziness and somnolence (drowsiness). “There was no evidence of withdrawal upon discontinuation of treatment with ALKS 5461.”
Overall, ALKS 5461 showed durability of antidepressant effect up to 52 weeks of treatment in patients with MDD. ALKS 5461 was well tolerated with an AE profile consistent with that reported in the short-term trials.
These results were repeated in Healio: “ALKS 5461 well-tolerated in major depression” and in Psychiatry Advisor: “Novel Buprenorphine Combinayion Therapy Shows Efficacy for MDD in Long-Term Trial.” Psychiatry Advisor has links to the Alkermes clinical trials referenced, which are all completed, but none had reported their results by June 1, 2018. Michael Thase’s report in Healio seemed to be in conflict with the research program noted above by FierceBiotech, unless he was counting the recently completed long term study. He said: “The research program for this compound has included a number of double blind studies, of which two are unequivocally positive; these data are being reviewed by the FDA for a possible indication as an adjunct to antidepressants.”
FierceBiotech pointed out where two of three clinical trials phase 3 clinical trails failed to meet their primary endpoints. And the third had its methodology and analysis modified after post-hoc analysis of the second clinical trial showed statistical significance in a subpopulation. Can the results be “unequivocally positive” when the primary endpoint for one of the positive studies was changed after it had begun?
There are some questions I have with regard to the assertion there was “no evidence of withdrawal upon discontinuation” for the long term study. What specifically were the researchers looking for as “evidence of withdrawal”? And when was the assessment done—immediately upon conclusion of the 52-week study?
Mood swings with bouts of anxiety or depression are common side effects with buprenorphine withdrawal. Granted, the buprenorphine dose is relatively small at 2 mg, but the time period of use at 52 weeks was long. “Buprenorphine withdrawal symptoms last longer for those who use buprenorphine for longer periods of time or at higher doses.” See “A Double-Edged Drug” for more on buprenorphine withdrawal.
However, psychological withdrawal symptoms can last for many months after cessation. It is recommended that you join a support group or see a psychologist who can help see you through the protracted or post acute withdrawal symptoms (PAWS). Many heavy buprenorphine users experience PAWS. With continued use of buprenorphine, there comes a point where the brain produces in an inadequate amount of neurotransmitters in the body. People going through buprenorphine PAWS manifest long lasting changes in the brain as a result of long term use. These changes are slower to reverse and can persist for many months, depending on the frequency and amount of past dosing.
Another thing to remember is that Suboxone (buprenorphine and naloxone) and ALKS 5461 (buprenorphine and samidorphin) appear to be biochemical twins. Also remember where employees of Alkermes have characterized buprenorphine as “addictive.” And the addictive potential of buprenorphine has not been entirely neutralized by its combination with Alkermes’ patented opioid antagonist, samidorphin (ALKS 33).
In “The Coming Depression Apocalypse,” I said I’m concerned the use of ALKS 5461 for treatment resistant depression would generate a population of individuals dependent upon buprenorphine. The problems coming off of ALKS 5461 would eclipse what we now know happens with SSRI withdrawal. A New York Times article said originally, antidepressants were supposed to be used short term for episodic mood problems—six to nine months. Now, almost 7% of Americans have been using antidepressants for at least five years. Patients who try to taper off antidepressants often find withdrawal symptoms are so severe they cannot stop. “Nearly half who tried to quit could not do so because of these symptoms.”
Coupling buprenorphine withdrawal in ALKS 5461 to the known issues with antidepressant withdrawal compounds the problems these medications were supposed to treat. And within the biochemical worldview, these symptoms will be reinterpreted as evidence of the underlying depression and proof the individual needs to remain on ALKS 5461 and/or antidepressants.




