The governor of Pennsylvania, Tom Wolf, declared the heroin and opioid epidemic as a statewide disaster emergency. Among the enhancements of the declaration, there will be increased access to the Prescription Monitoring Program so that state officials can identify doctors who are overprescribing opioid medication, as well as patients who may be seeing more than one physician to multiply their access to prescription opioids. Several measures to expand, speed up, and improve access to treatment and will be instituted. These measures include: enabling EMS to leave naloxone behind after responding to an overdose and expanding access to medication for Narcotic Treatment Programs. Pharmacists will be permitted to partner with prisons and treatment programs to make naloxone available to individuals leaving those facilities.
The Fix reported there was bipartisan support for the governor’s action. U.S. Senators, Pat Toomey (R) and Bob Casey (D) publically praised the declaration. Senator Toomey said: “The opioid and heroin crisis has rightfully drawn bipartisan attention in Congress and all levels of government. Today’s opioid emergency declaration sends a clear message that more work remains to be done.” Senator Casey added: “This declaration will bring additional resources to bear on this horrific public health emergency that has ripped apart far too many families.”
Increased access to the Prescription Monitoring Program by state officials is not as Orwellian as it may sound. Limitations placed upon the DEA by the “Ensuring Patient Access and Effective Drug Enforcement Act of 2016” hobbled the DEA’s ability to go after drug companies suspected of enabling the widespread distribution of prescription pain medication. “Overall, the drug industry spent $102 million lobbying Congress on the bill and other legislation between 2014 and 2016, according to lobbying reports.” See “Head of a Snake” for more information on this issue.
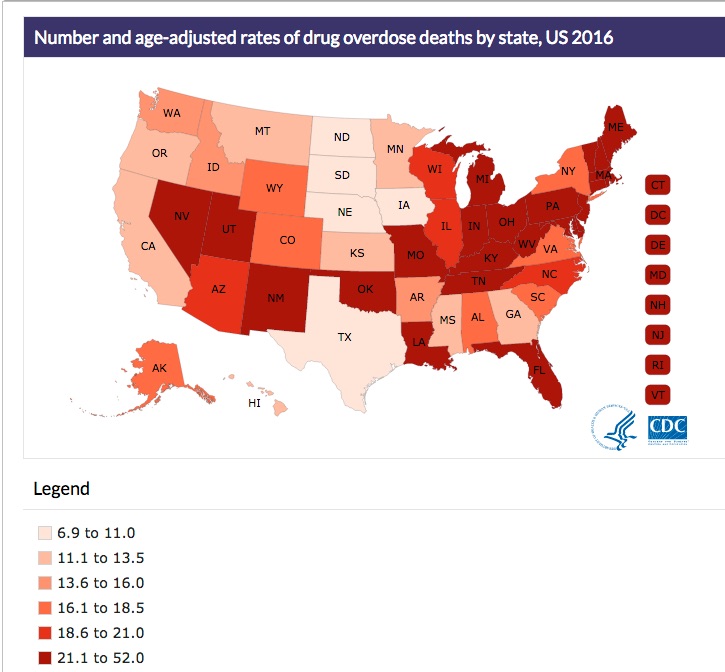
Drug overdose data from the CDC indicated that in 2016, Pennsylvania had the fourth highest increase in drug overdose deaths with 37.9 per 100,000. West Virginia (52.0 per 100,00), Ohio (39.1 per 100,00), New Hampshire (39.0 per 100,00) and Kentucky (33.5 per 100,000) rounded out the top five states. This was a 44.1% increase from 2015 to 2016 for Pennsylvania, again placing them fourth behind the increases with the District of Columbia (108.6%), Maryland 58.9%) and Florida (46.3%). New Jersey (42.3%) rounded out the top five states with percentage increases of overdose deaths. See the following graphic from the CDC report on rates of drug overdose death by state for 2016.
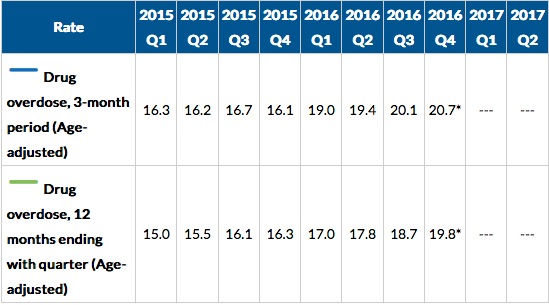
Another CDC report indicated the death rate for drug overdoses for the twelve-month period ending with the 4th quarter of 2016 was 19.8 per 100,000, an increase over the same time period for the 4th quarter of 2015, 16.3 per 100,000. This was an increase of 21% from 2015. The New York Times said Dr. Andrew Kolodny, the director of opioid policy research at Brandeis University, was not surprised by the data. “We have roughly two groups of Americans that are getting addicted. . . . We have an older group that is overdosing on pain medicine, and we have a younger group that is overdosing on black market opioids.” See the following table from the CDC report.
Naloxone, as it was noted in the opening paragraph, is a crucial tool in the struggle against opioid overdoses. Yet it had a quiet, and rather unassuming life until the rise of the opioid epidemic. Jack Fishman originally synthesized naloxone for a private narcotics lab owned by Mozes Lewenstein in 1961. Harold Blumberg, a colleague of Lewenstein’s, had the idea of developing an opioid antagonist by making a small structural change to oxymorphone, a synthetic opioid. The FDA approved naloxone as an injection, Narcan, to reverse opioid intoxication in 1971. Generic versions of naloxone became available in 1985.
As the opioid crisis began to pick up steam in 2013, the FDA approved Evzio, a portable injection kit with a fixed dose of naloxone. In late 2015, they approved Narcan, now packaged as a nasally administered form of naloxone. A series of governmental initiatives were enacted to increase access to naloxone. From 2012 to 2016, the number of states with at least one law expanding access to naloxone increased from 8 to 46. A growing number of community organizations now provide naloxone kits and education programs to laypersons. But as Gupta et al. reported in The New England Medical Journal, between 2009 and 2015 the annual number of naloxone prescriptions only increased from 2.8 million to 3.2 million. While retail-prescription numbers were unchanged, the proportion attributed to clinics and EMS providers increased from 14% to 29%.
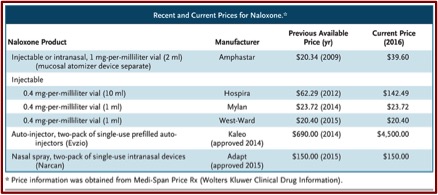
Although the slowed rate of using naloxone could be attributed to the stigma of opioid use and unfamiliarity with how to use naloxone, the rising cost and the limited number of manufacturers producing it, are more insidious reasons. While there are three manufacturers of naloxone approved by the FDA, there is only one supplier for all three formulations. Amphastar, the manufacturer of the 1-mg-per-millileter dose used off-label as a nasal spray, increased its price 95% to $39.60 in September of 2014. “Newer, easier-to-use formulations are even more expensive. Narcan costs $150 for two nasal-spray doses. A two-dose Evzio package was priced at $690 in 2014 but is $4,500 today, a price increase of more than 500% in just over 2 years.” See the following table of previous and current prices for naloxone.
 The price increase for naloxone is related to the overall trend of rising prescription drugs prices across-the–board. See “Pharma’s Not Getting the Message” and “Pharma Companies Hunt in Packs” for more information on this. But unfortunately, none of the federal or state initiatives to expand the availability of naloxone address the drug’s high price. “Evzio’s price jumped significantly and without explanation the month before the CDC’s coprescription guidelines were released.” Several U.S. senators have sent letters to naloxone manufacturers asking them to explain their price increases, but this hasn’t resulted in any changes or public outrage, as happened with Mylan, the manufacturer of the EpiPen. Gupta et al. had some recommendations to address naloxone’s price increase.
The price increase for naloxone is related to the overall trend of rising prescription drugs prices across-the–board. See “Pharma’s Not Getting the Message” and “Pharma Companies Hunt in Packs” for more information on this. But unfortunately, none of the federal or state initiatives to expand the availability of naloxone address the drug’s high price. “Evzio’s price jumped significantly and without explanation the month before the CDC’s coprescription guidelines were released.” Several U.S. senators have sent letters to naloxone manufacturers asking them to explain their price increases, but this hasn’t resulted in any changes or public outrage, as happened with Mylan, the manufacturer of the EpiPen. Gupta et al. had some recommendations to address naloxone’s price increase.
First, naloxone could be purchased in bulk, which would create stable demand that might motivate additional companies to begin manufacturing the medication — a strategy that’s been used for vaccine manufacturing. Second, governments could invoke federal law 28 U.S.C. section 1498 to contract with a manufacturer to act on behalf of the United States and produce less costly versions of Evzio’s patented auto-injector in exchange for reasonable royalties — an approach that was considered for procuring ciprofloxacin during the anthrax threat in 2001. Third, in response to increases in generic drug prices, some observers have proposed allowing importation of generics from international manufacturers that have received approval from regulators with standards comparable to those of the FDA, a strategy that could be pursued for naloxone.
Gupta et al. also suggested the federal government could motivate additional companies to obtain approval to market generic versions of naloxone by prioritizing timely approval and waiving application fees. This would likely stimulate price competition. Resurrecting a discussion of the FDA switching naloxone to over-the-counter-status would benefit patient access. “The relative ease of receiving FDA authorization for over-the-counter medications would also probably attract additional manufacturers.”
Naloxone coprescribing and expanded availability represents only one of many potential strategies for reducing the number of prescription-opioid and heroin overdose deaths in the United States. But when governments promote naloxone use, they have a responsibility to ensure the drug’s affordability. Taking action now is essential to ensuring that this lifesaving drug is available to patients and communities.
As illustrated within the actions taken by Governor Tom Wolf of Pennsylvania, naloxone is a key factor in the fight against the opioid epidemic. Given the potential influence of state and federal officials and legislators, concerted efforts to force manufacturers to decrease the cost of naloxone products or to take steps to increase their competition should be a no-brainer strategy. Otherwise—and this itself is another no-brainer—the pharmaceutical companies will continue to siphon as much profit as they can from the opioid epidemic.