In 2005, the FDA required atypical antipsychotics to carry a boxed warning that they had a higher risk of death related to use among individuals with dementia. In 2008, the FDA required the same warning with the older, typical antipsychotics. Antipsychotic use in nursing homes is now closely watched by government regulators, “who penalize and lower the ratings of facilities that overuse them.” This created an opportunity for the manufacturer of a little-used medication known as Nuedexta. Since 2012, there has been an increase of nearly 400% in the number of Nuedexta pills dispensed to long-term care facilities.
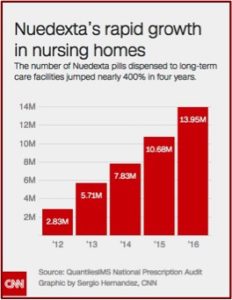
A CNN investigation reported on how “The maker of a little red pill intended to treat a rare condition is raking in hundreds of millions of dollars a year as it aggressively targets frail and elderly nursing home residents.” Nuedexta is approved to treat a disorder known as pseudobulbar affect or PBA. The disorder is a condition that causes sudden, uncontrollable crying and/or laughing. PBA afflicts less than 1% of all Americans and is most commonly found in people with multiple sclerosis (MS) or Lou Gehrig’s disease (ALS). The manufacturer of Nuedexta, Avanir, had its sales force focus on expanding the drug’s use with elderly patients struggling with dementia and Alzheimer’s disease. See the following graphic from CNN on the number of Nuedexta pills dispensed to long-term facilities.
Nuedexta is approved by the Food and Drug Administration (FDA) to treat anyone with PBA, including those with a variety of neurological conditions such as dementia. But geriatric physicians, dementia researchers and other medical experts told CNN that PBA is extremely rare in dementia patients; several said it affects 5% or less. And state regulators have found doctors inappropriately diagnosing nursing home residents with PBA to justify using Nuedexta to treat patients whose confusion, agitation and unruly behavior make them difficult to manage.
CNN identified dozens of examples across the country where the use of Nuedexta was questionable. In one Los Angeles nursing home, regulators found that 28% of its 162 residents had been placed on Nuedexta. A facility psychiatrist, who is a paid speaker for Avanir, had given a talk about the drug to nursing home employees. An employee at another Southern California facility admitted a resident was given a diagnosis of PBA in order to justify the use of Nuedexta, “even though its intended purpose was to control the resident’s “mood disturbances” and yelling out.” An Ohio doctor has come under investigation for allegedly receiving kickbacks for fraudulently diagnosing PBA in order to get Medicare coverage for Nuedexta.
The federal government foots the bill for a big portion of the money being spent on Nuedexta in the form of Medicare Part D prescription drug funding, for people 65 and over and the disabled. In 2015, the most recent year for which data is available, this Medicare program spent $138 million on Nuedexta — up more than 400% from just three years earlier.
The only FDA approved medication for patients with PBA is Nuedexta. “So experts say that Medicare coverage of the drug, which has been crucial to its financial success, relies on the diagnosis of this single condition.” Off-label prescribing of Nuedexta to treat patients with behavioral issues who were not diagnosed with PBA would likely not be covered. CNN analysis found that almost half of the Nuedexta claims filed with Medicare in 2015 “came from doctors who had received money or other perks from the company [Avanir].”
During the FDA approval process, two doctors on the committee raise concerns about Nuedexta being used for PBA in Alzheimer’s patients. “They both strongly recommended that Nuedexta only be approved for PBA in patients with MS or ALS.” Evidence, they argued, that it would be effective in other conditions was weak; and not enough was known about the safety of the drug in the elderly. Despite these concerns, the FDA did not limit its use as recommended. “The agency approved Nuedexta in 2010 for treating PBA in patients who have neurological conditions such as dementia.”
Soon after it hit the market, Neudexta was getting reports of potential harm, ranging from dizziness and falls to coma and death. “Nuedexta was listed as a ‘suspect’ medication in nearly 1,000 so-called adverse event reports received by the FDA detailing side effects, drug interactions and other issues.” It was listed by caregivers as a potential cause of 133 hospitalizations (which included 8 cases that also resulted in death), 51 deaths, 102 incidents of sedation and sleepiness and 101 of dizziness, confusion and falls. A nurse practioner reported on an 86-year-old Alzheimer’s patient who rapidly declined after Nuedexta was added to her Zoloft (an antidepressant), Xanax (an antianxiety medication) and Risperidone (an antipsychotic). Almost immediately she declined and became almost unresponsive, ultimately dying.
Nuedexta is a combination of two generic drugs, dextromethorphan and quinidine that are respectively a cough suppressant and antiarrhythmic drug (it slows rapid heartbeat and helps your heart beat regularly). They were once available from specialty pharmacists for less than $1 a pill. The FDA-approved medication now costs as much as $12.60 a pill; more than $9,000 a year. “Medicare Part D spending on the drug averaged $3,400 per patient in 2015.” It gained public attention through a television commercial featuring actor Danny Glover cycling through fits of laughing and crying.
Avanir executives have wanted to secure FDA approval for Nuedexta’s use to treat dementia patients who don’t have PBA, “setting their sights on the more widespread condition of agitation in dementia and Alzheimer’s patients, characterized by emotional and physical outbursts and restless behaviors.” A study has been completed that looked at the efficacy, safety and tolerability of Neudexta for the treatment of agitation in Alzheimer’s patients, but the results haven’t been posted. “Without additional FDA approval for the drug’s use in those conditions, salespeople cannot promote Nuedexta for that purpose. They can only market its use for dementia patients who also have PBA.”
Across the country, the use of Nuedexta in nursing homes has prompted concerns among state regulators whose job is to ensure adherence to federal guidelines and protect residents from being given unnecessary drugs — especially those used as chemical restraints. But to date, the red flags raised by these regulators have been largely left buried in nursing home inspection reports and have drawn little public attention. CNN identified more than 80 cases in 19 states since 2013 where inspectors cited nursing homes for inappropriate monitoring and use of Nuedexta — often because residents hadn’t exhibited any symptoms of PBA. Many of the cases — about 40% — were clustered in Southern California, where Avanir is based and where former employees said there has been aggressive marketing.
Currently there are no FDA-approved drugs to treat dementia-related agitation. But those who care for the elderly are eager to find tools to manage agitated and aggressive patients. Staff increases could reduce the need for medications. But these measures don’t always work and they are expensive. So some facilities use pharmaceuticals to help make their patients easier to treat. Helen Kales, a geriatric psychiatrist said: “”Rather than taking someone off an antipsychotic … providers search for a different ‘magic bullet.'”
McKnight’s Long-Term Care News reported that The Centers for Medicare & Medicaid Services said that it met its goal of reducing the national rate of antipsychotic use among long-term residents by 20% by the end of 2016. However, Winter et al. reported in Clinical Gerontologist there was a 12% increase for the combined rates of schizophrenia, Tourette’s and Huntingdon’s among long-term residents. The increase began in 2012 at a rate almost triple that of the general long-term nursing home population. Most of the increase was in the rates of schizophrenia reported. The increased “identification” of these psychiatric conditions seems new “and concentrated in residents on antipsychotics.”
Since antipsychotics prescribed for schizophrenia, Tourette’s, and Huntington’s are excluded from quality-measure auditing, apparent reductions in inappropriate long-stay antipsychotic use since the National Partnership may be exaggerated.
Sadly it seems that despite the clear evidence of adverse effects from antipsychotics and Nuedexta with the elderly, long-term care facilities have simply conjured up a diagnosis to justify their use.