Not the End of Smoking

America’s teens reported a striking increase in their vaping in the previous 12 months, from 27.8 % in 2017 to 37.3% in 2018. Reported vaping in the 30 days prior to the survey almost doubled among high school seniors, going from 11% in 2017 to 20.9% in 2018. Nora Volkow, the director of the National Institute on Drug Abuse (NIDA), said: “Teens are clearly attracted to the marketable technology and flavorings seen in vaping devices; however, it is urgent that teens understand the possible effects of vaping on overall health; the development of the teen brain; and the potential for addiction.” She went on to say research shows that teens who vape may be at risk of transitioning to smoking cigarettes.
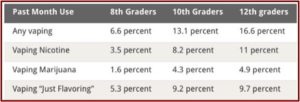
The increase in vaping rates for 2017-2018 was consistent with the findings of the National Youth Tobacco Survey, which said e-cigarettes were the most commonly used tobacco product among U.S. middle school and high school students. The most selected reason for e-cigarette use was they were available in flavors like mint, candy, fruit or chocolate. The lead author for the Monitoring the Future study, which annually surveys 12th, 10th and 8th grade students, said in a letter to The New England Medical Journal the increases in the prevalence of vaping nicotine translated into roughly 1.3 million additional adolescents vaping.
Put in historical context, the absolute increases in the prevalence of nicotine vaping among 12th-graders and 10th-graders are the largest ever recorded by Monitoring the Future in the 44 years that it has continuously tracked dozens of substances. These results indicate that the policies in place as of the 2017–2018 school year were not sufficient to stop the spread of nicotine vaping among adolescents. The rapid entry of new vaping devices on the market, the latest example of which is the Juul, will require continual updates and modification of strategies to keep adolescents from vaping and its associated negative health effects.
Scott Gottlieb, the former commissioner of the FDA, who had been active in efforts to thwart this trend, sent 1,300 warning letters over the summer of 2018 to online and brick-and-mortar stores for selling e-cigarette products to underage consumers. He also sent out warning letters to five manufacturers, asking them to address the popularity of their products with underage consumers or risk the removal of “some or all of their flavored products.” In the letter sent to Juul Labs, the FDA requested that within 60 days, “you provide a written response to this letter that includes a detailed plan, including specific timeframes, to address and mitigate widespread use by minors.” Giving suggestions for that plan, the letter said Juul Labs could:
- Discontinue sales to retail establishments that have been subject to an FDA civil monetary penalty for sale of tobacco products to minors within the prior 12 months;
- Develop or strengthen any internal program you have to check on retailers, and report to FDA the name and address of retailers that have sold products to minors;
- Eliminate online sales, whether through Internet storefronts controlled by your company or other retailers, or provide evidence to demonstrate that your company’s online sales practices do not contribute to youth use of JUUL products;
- Revise your current marketing practices to help prevent use by minors;
- Remove flavored products from the market until those products can be reviewed by FDA.
Then unexpectedly, in the middle of his crusade against teen vaping, Scott Gottlieb resigned as the commissioner of the FDA, effective April of 2019. His stated intention was to spend more time with his wife and three daughters. He said: “There’s perhaps nothing that could pull me away from this role other than the challenge of being apart from my family for these past two years and missing my wife and three young children.” Gottlieb said the policies he initiated will move forward as planned, including the proposed restrictions recently announced on the sale of flavored e-cigarette products. The New York Times said retailers were on Capitol Hill lobbying against the FDA proposals. “Conservative groups and vaping trade associations also have come out in opposition, saying that the agency’s efforts to regulate the e-cigarette industry amount to government overreach.” Yet some public health advocates think the action steps were too late.
A December 2018 investigation reported in JAMA Network Open sought to estimate the concentration of tobacco-related toxins among e-cigarette users. Findings suggested that exclusive e-cigarette use resulted in measurable exposure to tobacco-related toxicants, generally at lower levels than with cigarette smoking. Dual users had higher concentrations of exposure to nearly all biomarkers when compared with cigarette-only smokers. “Several biomarkers measured in this study are metabolites of known carcinogens as well as respiratory, cardiovascular and/or reproductive/developmental toxicants.” The data clearly showed e-cigarette users were exposed to known tobacco-related toxicants.
Another study presented at the American College of Cardiology’s 68ith Annual Scientific Session shoed that adults using e-cigarettes are significantly more likely to have a heart attack, coronary disease and depression when compared to nonusers. The study’s lead author said: “Until now, little has been known about cardiovascular events relative to e-cigarette use. These data are a real wake-up call and should prompt more action and awareness about the dangers of e-cigarettes.”
This study found that compared with nonusers, e-cigarette users were 56 percent more likely to have a heart attack and 30 percent more likely to suffer a stroke. Coronary artery disease and circulatory problems, including blood clots, were also much higher among those who vape — 10 percent and 44 percent higher, respectively. This group was also twice as likely to suffer from depression, anxiety and other emotional problems.
Scott Gottlieb said that if the 2019 National Youth Tobacco Survey shows another spike in teen use of e-cigarettes, “We’re going to be back making new policy in the fall.” One of those possible changes could be taking all pod-based e-cigarettes off the market. “At some point, the youth use of those products becomes so intolerable that they have no redeeming public health value, and we’ll just have to sweep the market of those products. And that includes Juul. Those are the products being abused by the children.”
In a statement released on Wednesday, March 13, 2019, Juul Labs said they are committed to reducing youth usage of their products, while preserving their opportunity to “eliminate combustible cigarettes.” They noted how in November of 2018 they stopped the sale of flavored Juul pods to retail stores, strengthened their retail compliance and secret shopper program, enhanced their online age-verification and exited their Facebook and Instagram accounts.
We support category-wide action including the responsible, restricted sale of flavored products and will review today’s draft guidance as we continue to work with FDA, state Attorneys General, local municipalities, and community organizations as a transparent and responsible partner in combating underage use.
So, what is the bottom line on the risks of e-cigarettes for teens? The CDC has a page of “Quick Facts on the Risks of E-Cigarettes.” It said the use of e-cigarettes in not safe for teens and young adults. They contain nicotine, which is highly addictive “and can harm the adolescent brain development.” Young people who use e-cigarettes may also be more likely to smoke cigarettes in the future, which points to the motive underlying Gottlieb’s crusade.
Finally, what about Juul Labs desire to use its e-cigarette to eliminate combustible cigarettes? One of the strongest arguments for vaping is that it can help people taper off from their addiction to tobacco smoking. A group of researchers sought to generate evidence on the ‘real world’ use of e-cigarettes on the quit rates of adult smokers. They recruited 1284 current, established smokers and then re-contacted them after a year. Although 16% had stopped smoking, they found no evidence that electronic nicotine delivery systems (or ENDS) were helping adults quit “at a higher rate than smokers who did not use these products, despite ENDS users being more likely to make a quit attempt.” In other words, they did not put an END to smoking.
Our study suggests that use of current ENDS products in real world conditions do not seem to improve the chances of quitting for smokers, and, under the current landscape, may not be the disruptive technology that increases the population quit rate and reduces the harm of combustibles. Additional steps may be needed to spur innovation to create low-harm and low-risk products that adequately deliver nicotine, address the misperceptions of relative harm of ENDS compared to cigarettes, and encourage cessation and complete switching from combustibles to low-harm and low-risk products among smokers who do not want to quit smoking.
Changes to their design, to how e-cigarettes are marketed and regulated may be too slow and ineffectual to lead to the above changes on their own, according to the lead author of the ‘real world’ study published in PLoS One. But for now, it seems e-cigarettes are more likely to become an add-on to smokers than an effective tool to stop smoking. “People who use both tobacco and e-cigarettes are actually less likely to quit smoking than people who only stick to tobacco.”