Not as a Stand-Alone Therapy

The pharmaceutical company Alkermes began planning an alcohol dependency awareness campaign before the pandemic, but its launch in May of 2021 coincidently happened to overlap with the publicity of increasing of alcohol use and misuse during the pandemic. Fierce Pharma noted that while all drinking is not problematic, spikes in binge drinking particularly among women, are causes for concern. The Alkermes campaign emphasizes that alcohol dependence is a disease, not a moral failing. The VP and general manager of addiction marketing at Alkermes said, the disease model was useful for people to see problem drinking as a medical condition, “that there are medical criteria that you can assess and look at your own situation and make informed choices.”
The campaign initially centered on a new website that asks on the front page, “Is it time to rethink your relationship with alcohol?” There are personal stories of people’s “journey with alcohol dependence and recovery.” There is a link to a questionnaire developed by the National Institutes of Health (NIH) that was based on the criteria for alcohol dependence outlined in the DSM-IV. Finally, there is a link to “Learn more about a treatment option,” which leads you to a separate website for Alkermes’ addiction drug, Vivitrol. But there is more going on here than just following a breadcrumb trail from a public service campaign to a treatment option for alcohol dependence.
If you follow the link to the Vivitrol website, you are asked if you are ready for the next step in your recovery. What follows is largely a repeat of information that is contained in the medication guide for Vivitrol. Even though this Alkermes ad campaign is aimed to treat alcohol dependence, there is a significant amount of time spent there describing and discussing the risk of opioid overdose and sudden opioid withdrawal. What you won’t find on the website is information on exactly how Vivitrol treats alcohol dependence! Why would a marketing campaign for an alcohol dependence treatment spend so much effort cautioning against how Vivitrol could create safety issues with opioids?
Because Alkermes has been known to gloss over safety risks in its promotion of Vivitrol to treat opioid addiction. Not only is Vivitrol used to treat alcohol dependence, it is also approved as a medication-assisted treatment for opioid use disorder.
The FDA previously cited Alkermes in a warning letter for omitting safety risks in an ad for Vivitrol. “In the letter, the agency said it had contacted the company twice before issuing the warning.” The FDA was concerned enough with Alkermes’ apparent disregard of it previous letters that it also issued a press release that announced this action. The press release said Alkermes had omitted warnings about the most serious risks associated with the drug from promotional materials.
While the print advertisement contains claims and representations about the drug’s benefits, it fails to adequately communicate important warnings and precautions listed in the product labeling, including vulnerability to opioid overdose, a potentially fatal risk.
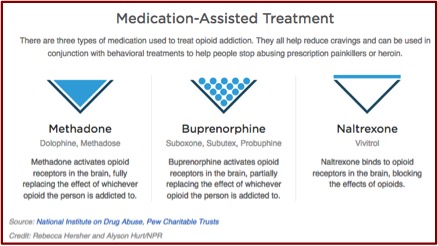
What you won’t find on the new Vivitrol website or in the Vivitrol medication guide is information on exactly how it treats alcohol dependence and what are the specific concerns in using Vivitrol to treat alcohol dependence. The active ingredient in Vivitrol is naltrexone, which has been used to treat alcohol use disorder for over twenty years. It works by suppressing cravings for alcohol and opioid drugs. It does this by binding to opioid receptors in the person’s brain, which also removes any opioid drugs binding with these receptors and can precipitate sudden opioid withdrawal. The medication guide for Vivitrol cautions that anyone receiving the drug should be opioid-free for at least 7 to 14 days before receiving Vivitrol, since the injection “may cause you to suddenly have symptoms of opioid withdrawal.”
The American Addiction Centers website said the following about naltrexone and naltrexone’s effectiveness:
Individuals with moderate to severe alcohol use disorders [i.e., alcohol dependence] who are using naltrexone may experience withdrawal symptoms if they stop drinking that can be potentially fatal due to the development of seizures. These individuals should consult with an addiction medicine physician or psychiatrist before discontinuing their use of alcohol. Research findings are mixed, but overall, they tend to support the notion that individuals who use naltrexone to treat alcohol abuse reduce the total amount of alcohol they consume and observe a reduction in the number of times they drink alcohol. In addition, heavy drinkers often notice significant reductions in alcohol use. However, the research does not indicate that the use of naltrexone is effective at assisting individuals in remaining totally abstinent, but it does most likely result in a significant reduction in cravings for alcohol and an overall reduction in the amount of alcohol consumed.
Counterintuitively, naltrexone products like Vivitrol are not effective in helping someone abstain from alcohol. But as the American Addiction Centers website noted, it is used in the Sinclair Method to help the individual reduce their alcohol intake. This “treatment” method actually encourages individuals to drink, but only after taking naltrexone before they start drinking. Naltrexone blocks endorphins from being released when alcohol is consumed. Endorphins are naturally occurring opiates in the brain. “When the endorphins are blocked, there is no ‘buzz’ or rewarding experience, and the alcohol doesn’t make you feel the pleasure that drives you to drink excessively.”
The Sinclair Method sees alcoholism as primarily a learned behavior that can be extinguished by naltrexone systematically closing off this reward circuit in the brain. But naltrexone (as ReVia or Vivitrol) does not close the door on how alcohol effects functional impairments, such as a loss of motor coordination, decreased response time, slowed rate of thinking and judgement. ReVia is a tablet or capsule of naltrexone that you take about an hour before you plan to drink and can be skipped if you plan on drinking and want to feel the euphoria from drinking. With Vivitrol, you have some level of naltrexone in your system for up to a month, meaning that the blocking effect when drinking is stronger the closer you are to when you received your Vivitrol shot.
Vivitrol may be effective in reducing your overall alcohol intake when drinking, but it does not seem to be effective in helping you remain totally abstinent. Moreover, if your alcohol intake is substantial enough, and Vivitrol successfully helps you reduce your craving for alcohol and the amount of alcohol you consume too rapidly, using it can result in seizures and other alcohol withdrawal symptoms. If you use or abuse opioids along with drinking alcohol, Vivitrol can also throw you into sudden opioid withdrawal. It also decreases your tolerance level for opioids and makes you vulnerable to opioid overdose, if you use opioids.
Above, I noted where the Alkermes campaign for Vivitrol as a treatment for alcohol dependence emphasized it was a medical condition with criteria that could be assessed by an individual as they make an informed choice on whether Vivitrol was right for them. Alcohol dependence is never just a medical condition. And recovery is not simply learning to abstain or drastically curtail your alcohol use. It is also about making radical changes in your feeling, thinking and behavior around alcohol. To its credit, Alkermes did emphasize that for Vivitrol to be effective, it must be used with other alcohol or drug recovery programs. So, Vivitrol may work in some cases with alcohol abuse, but not as a stand-alone therapy for alcohol dependence.
Remember the final advice Alkermes gives: “Vivitrol may not work for everyone.”