The American Heart Association recently published a scientific statement on the use of e-cigarettes, “Cardiopulmonary Impact of Electronic Cigarettes and Vaping Products.” It describes the latest usage trends, the current scientific evidence about e-cigarettes and identifies current health impacts. It noted that vaping and e-cigarette use has grown exponentially over the past ten years, particularly among youth and young adults. They have been touted as safer alternatives to tobacco cigarettes, and even as potential tobacco-cessation products. However, e-cigarettes in 2019 led to more than 2,800 hospitalizations.
The CDC reported that as of February 18, 2020, a total of 2,807 hospitalized EVALI (e-cigarette or vaping product use-associated lung injury) cases or deaths were reported. Laboratory data showed that vitamin E acetate, an additive in some THC-containing products, was strongly linked to the EVALI outbreak. The CDC and FDA recommended that people not use THC-containing ENDS—electronic nicotine delivery system. After the identification of the primary cause of EVALI, and a significant decline in EVALI cases, the CDC stopped collecting data from states as of February 2020, the beginning of the COVID pandemic.
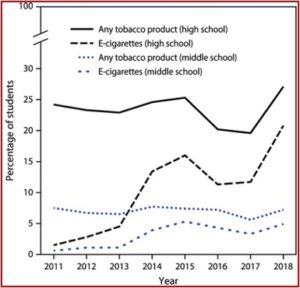
By 2019 in the U.S., 27.5% of high school students said they used e-cigarettes or ENDS. These products are the most commonly used tobacco products among youth, a growing number of whom reported never smoking combustible cigarettes. Data from the National Youth Tobacco Survey (NYTS) indicated current use in the past 30 days of ENDS increased from 1.5% in 2011 to 20.8% in 2018, an estimated 3.1 million students. Among middle school students, current e-cigarette use increased from .6% in 2011 to 4.9% in 2018, an estimated 570,000 students. See the following figure from “Cardiopulmonary Impact of Electronic Cigarettes and Vaping Products.”
Data from the 2019 NYTS indicated 25.5% of 12th graders reported current e-cigarette use compared to 11% in 2017. Current THC (cannabis) vaping increased in 12th graders from 4.9% in 2017 to 14.0% in 2019, 4.3% to 12.6% with 10th graders, and 1.6% to 3.9% with 8th graders. The prevalence of ENDS use among youth remained stable despite the pandemic. Data from 2020 showed ENDS use declined to 19.6% among high school students and to 4.7% among middle school students. “Whether this is an artifact of the great societal disruptions from the global pandemic or represents a decreased trend remains to be seen.”
Because of the rapid evolution of ENDS, it is important to examine prevalence rates with other vaping products besides e-cigarettes such as e-hookahs (e-waterpipes). E-hookahs are a new category of vaping devices, introduced in 2014 and recently patented by Philip Morris [the tobacco company], that are marketed as healthier alternatives to traditional hookah fruit-flavored tobacco smoking. Findings from the nationally represented PATH study (Population Assessment of Tobacco and Health; 2014–2015) in children 12 to 17 years of age indicated that 7.7% were identified as ever-users of e-hookahs compared with 14.26% who were ever-users of ENDS products.
Studies in the U.S. indicate a rapid increase in ever and current ENDS use among adults since 2010, “with the vast majority of users being current or former cigarette smokers.” Recent analysis of NHIS (National Health Interview Survey) data from 2014 to 2018 showed young adults 18 to 24 years of age are using ENDS at high rates. Current use increased from 5.1% to 7.6%. There were large increases among never-smokers (1.5% to 4.6%) and former smokers (10.4% to 36.5%).
See “Cardiopulmonary Impact of Electronic Cigarettes and Vaping Products” for a detailed discussion of the acute health effects and toxicity of e-cigarettes and vaping products.
Chronic Health Effects and Toxicity of E-Cigarettes and Vaping Products
E-cigarettes were created in China in the early 2000s and introduced to the US market in 2007. The basic mechanism heats or atomizes a liquid solution or e-liquid that generally contains a humectant (a substance used to keep things moist), nicotine and flavoring agents. The e-liquid formulations can contain other drugs beside nicotine, including THC, methamphetamine and methadone. The FDA attempted to stop the importation of these products, recognizing they could be used as drug-delivery devices. But a 2010 court ruling, Smoking Everywhere, Inc. vs US Food and Drug Administration deemed e-cigarettes should be considered tobacco products, and fall under the 2009 Family Smoking Prevention and Tobacco Control Act.
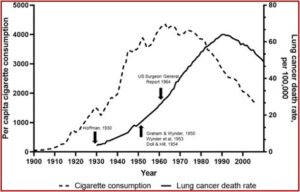
E-cigarettes and vaping were introduced in the US 16 years ago, and only saw widespread adoption in the past ten years. “We do not yet know the long-term health effects of these products.” Tobacco use was not recognized as a major preventable cause of death until many years after cigarette smoking became widespread. An increasing incidence of lung cancer was not noted until 1930. Definite scientific evidence associating cigarette smoking and lung cancer was not reported until the 1950s.
In 1964, the US Surgeon General report on tobacco and health attributed the increase in lung cancer to cigarette smoking. Only then did cigarette smoking per capita begin to decline. With the delayed development of chronic disease from smoking, lung cancer deaths did not begin to fall accordingly until decades after the 1964 report.
See the following figure taken from “Cardiopulmonary Impact of Electronic Cigarettes and Vaping Products.”
In 2018 an evidence-based summary of the health concerns with ENDS found no available evidence that ENDS use was associated with coronary heart disease, stroke, and peripheral artery disease. There was insufficient evidence that ENDS use was associated with long-term effects on heart rate, blood pressure and cardiac function. There was also no available evidence on whether ENDS use causes respiratory diseases in humans. There was only moderate evidence ENDS use is associated with increased asthma problems, and limited evidence of adverse effects of ENDS exposure on the respiratory system.
There have been few studies of the chronic cardiovascular effects of ENDS because they have only been available for the past 16 years! Assuming similar time delays for the appearance of chronic disease from cigarettes and for ENDS, “epidemiological increases in disease prevalence would not be expected to be observed for years.”
Vaping devices have not been shown to be safe for long-term use. The short- and long-term toxicities of inhaling aerosols generated from liquids containing vegetable glycerin, propylene glycol, nicotine, or flavors are unknown. Inhaling aerosols generated from THC- or CBD-containing liquids, which often contain additional chemical components, also have unknown health effects. Thus, elucidating their long-term respiratory, cardiac, and cancer health effects is a public health priority.
E-Cigarette and Vaping Products as Cigarette-Cessation Products
The Cochrane Review found that nicotine e-cigarettes can be effective in helping people stop smoking for at least six months. They were found to be more effective than nicotine replacement therapy and cessation with e-cigarettes without nicotine. And yet, they strongly discouraged those who have never smoked from using e-cigarettes, especially young people. “This is because they are a relatively new product and we don’t yet know the long-term health effects.”
“Cardiopulmonary Impact of Electronic Cigarettes and Vaping Products” said in the Cochrane Review adverse events were higher at 12 weeks to 6 months in ENDS users when compared to no support or behavioral support only. One study compared e-cigarettes with varenicline (Chantix), finding e-cigarettes were less effective than varenicline. Four of 27 e-cigarette users versus 13 of 27 varenicline users stopped smoking. The main study results assessed smoking cessation and not complete product cessation. “This could mean that participants who quit smoking continued ENDS use.”
Another study compared nicotine ENDS plus a nicotine patch (NRT) with NRT alone and NRT plus nicotine-free ENDS. The patch alone has a 2% abstinence rate. The patch and nicotine-free ENDS had a 4% abstinence rate and the nicotine patch and nicotine ENDS had a 7% abstinence rate. “No current ENDS products have FDA approval as a tobacco-cessation aid. There is only low to moderate confidence of improved cessation with nicotine-containing ENDS products compared with NRT or behavioral interventions.”
There are few empirically tested prevention and cessation programs for youth ENDS use. Using novel technology—text messages, social media—that have been used extensively to advertise ENDS products to youth, as wells as educational efforts targeting parents and health educators, and other methods have been shown to promote smoking cessation among youth. But further work is needed to develop and test effective interventions.
Conclusion
ENDS products have undeniably been increasing in popularity, particularly among young adults and teens, in the past decade. The constituents of these products often include nicotine, which is well established to have negative health effects and strong addictive properties. Other ingredients, particularly in flavored products, have known health risks. Because ENDS products are not regulated as classic therapeutic drugs or devices, there are no dedicated long-term safety studies. Critical questions remain unanswered about the short-term and, in particular, long-term health effects of ENDS products. Because the products have only recently gained widespread use, decades of prospective or retrospective data are not yet available to examine the long-term health effects of cigarettes. Early analysis suggests some utility of ENDS as a smoking cessation product; however, any benefit needs to be juxtaposed with a clear understanding of the health risks of the ENDS products themselves and the risks of product availability leading to nonsmokers initiating ENDS use.