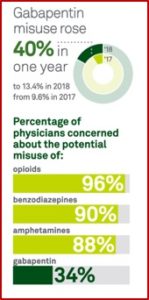
Quest Diagnostics generated more than $7.7 billion in revenue in 2017 by offering diagnostic testing services for cancer, cardiovascular disease, infectious disease, neurological disorders and employment and drug testing. The company recently published a report of prescription drug misuse in the U.S. based on analysis of clinical drug monitoring through Quest and a survey of 500 primary care physicians. It found that gabapentin was emerging as an alternative pain therapy to opioids while its misuse rose 40% in one year—13.4% in 2018 from 9.6% in 2017. “This makes gabapentin the most commonly misused prescription drug in 11 states and in the top three drug groups in an additional 10 states.”
The report presented findings from over 4.4 million drug monitoring tests for patients in all 50 states and the District of Columbia from 2011 through 2018. Physicians thought gabapentin was a less risky alternative to opioids and are less concerned about its potential for misuse. The survey found that in an effort to avoid prescribing opioids, 78% of physicians prescribed gabapentin to their patients with chronic pain and 85% had done so in the past six months. Sixty-three percent believed less than 10% of patients prescribed gabapentin misuse it. Concern for the potential of gabapentin misuse was significantly lowers than opioids, benzodiazepines and amphetamines. See the graphic from the Quest report.
 When taken alone and as prescribed, there is less of a risk for misuse or addiction. Yet taking gabapentin with other medications such as muscle relaxants, opioids and anxiety medications can produce a high. Vice reported the FDA has only approved gabapentin for treating seizures and nerve pain from shingles, but its reach extends far beyond those two conditions. It is prescribed for migraines, fibromyalgia, hot flashes, depression, bipolar disorder, restless leg syndrome, anxiety, and a variety of other nerve and chronic pain issues. Jordan Covvey, an assistant professor of pharmacy at Duquesne University, said “It’s the ‘lets’ just throw something at the wall and hope that it magically sticks’ drug.” She added that a lot of damage could be happening with that sort of strategy.
When taken alone and as prescribed, there is less of a risk for misuse or addiction. Yet taking gabapentin with other medications such as muscle relaxants, opioids and anxiety medications can produce a high. Vice reported the FDA has only approved gabapentin for treating seizures and nerve pain from shingles, but its reach extends far beyond those two conditions. It is prescribed for migraines, fibromyalgia, hot flashes, depression, bipolar disorder, restless leg syndrome, anxiety, and a variety of other nerve and chronic pain issues. Jordan Covvey, an assistant professor of pharmacy at Duquesne University, said “It’s the ‘lets’ just throw something at the wall and hope that it magically sticks’ drug.” She added that a lot of damage could be happening with that sort of strategy.
Recent studies have questioned gabapentin’s use as a benign catch-all drug and documented its potential for misuse. There is an increased risk of death when it is combined with opioids and a worrying correlation with suicide. Despite the concerns, its use continues to rise. IMS reported gabapentin prescriptions increased from 39 million in 2012 to 64 million in 2016, making it the 10th most prescribed medication. Lyrica (pregabalin), another gabapentinoid, ranked 8th by invoice spending.
In 2017 Christopher Goodman and Allan Brett said in the New England Medical Journal that gabapentinoids (gabapentin and pregabalin) were being overprescribed. The FDA approved gabapentinoids for treating postherpetic neuralgia (gabapentin and pregabalin), fibromyalgia (pregabalin), and neuropathic pain associated with diabetes or spinal cord injuries (pregabalin). But they are being increasingly prescribed for almost any type of pain.
We found that most recently published clinical studies of gabapentinoids for pain examined single-dose or short-course gabapentinoids for mitigating postoperative pain, an indication that isn’t relevant to general outpatient practice. Relatively few clinical trials have assessed the use of gabapentinoids in the common pain syndromes for which they are prescribed off-label — and many of those trials were uncontrolled or inadequately controlled and of short duration. Among the few well-conducted, properly controlled, double-blind studies, results have been mixed at best.
An estimated 95% of gabapentin prescriptions are for off-label use. According to Joe Ross, a researcher on pharmaceutical policy, there are no well-designed, placebo-controlled clinical trials for several of its off-label uses. Some have one or two studies, but the results are either modest or inconsistent. “Only about 20 percent of gabapentin’s off-label uses have data supporting them.” See the Vice article and “Twentieth Century Snake Oil” for a description of how Parke-Davis, the company that brought Neurontin (gabapentin) to market spent millions of dollars on a deceptive marketing campaign to promote gabapentin’s off-label use. Doctors were said to reach for gabapentin in situations where someone is difficult to treat.
Another concern noted by Goodman and Brett was the misuse and abuse of gabapentin and pregabalin. They cited “Abuse and Misuse of Pregabalin and Gabapentin,” which said an increasing number of patients are self-administering higher than recommended doses of gabapentinoids to achieve euphoric highs. “In the general population, a 1.6% prevalence of gabapentinoid abuse was observed, whereas prevalence ranged from 3% to 68% among opioid abusers.” They concluded the evidence suggests particularly in individuals with a history of opioid abuse, gabapentinoids have a potential for abuse. Another study of opioid users in Kentucky reported 15% of participants used gabapentin specifically “to get high” in the past six months. This was a 165% increase from the year before.
Peckham, Ananickal and Sclar said the abuse potential of gabapentin was well documented and it was highly sought after for use in potentiating opioids. While the US was in the midst of an opioid epidemic, the national focus has overshadowed the growing diversion and concomitant abuse of prescription medications like gabapentin to potentiate an opioid high. “Gabapentin presents as an opportunistic prescription drug of abuse, given its relatively low cost and non-schedule status at the federal level.”
They reported that 24% of patients with sustained co-prescription of gabapentin and opioids had at least three prescription claims exceeding the established dosage thresholds. “This is of particular concern, as abuse of gabapentin in concert with opioids has been associated with a fourfold increased risk of respiratory depression, the primary cause of death in opioid-related overdose.” Research suggested that when gabapentin exceeded a dose of 900 mg, it could lead to a 60% increase in the odds of opioid-related death relative to the abuse of opioids alone.
In the absence of federal efforts to reclassify gabapentin as a controlled substance, a small number of US states have implemented a number of regulatory approaches to mitigate diversion and abuse. Primary strategies include the reclassification of gabapentin as a controlled substance and mandating the reporting of the prescribing and/or dispensing of gabapentin to a state-level PDMP. These efforts are progressive both nationally and globally, as gabapentin is not classified as a controlled substance in Europe despite previous European reports of gabapentin abuse, nor is it a controlled substance in Australia or Canada.While state-level efforts to combat the diversion and abuse of gabapentin, and thus the opioid epidemic, are to be commended, such efforts are not a substitute for a strategic national approach. Given the growing empirical evidence surrounding both the diversion and abuse of gabapentin, we call for reclassification as a controlled substance at the federal level and implementation of a national pharmacovigilance program. Additionally, future research is needed to identify the degree of regulatory oversight needed to effectively detect and mitigate gabapentin abuse.
A study published in Clinical Toxicology examined the misuse and toxicology trends associated with gabapentin and baclofen, using data gleaned from the National Poison Data System (NPDS). From 2013 to 2017 all gabapentin exposures increased by 72.3%. All fifty states saw an increase in exposure to gabapentin. The authors also noted that misuse and diversion of gabapentin has been well-documented. Intentional suicide attempts with gabapentin increased by 80.5%. The authors noted the increased exposures coincided with reductions in opioid prescribing. They speculated the increases may represent an unintended consequence of the need for effective pain management and the migration away from opioid use.
Vice reported that while gabapentin is not classified as a controlled substance at the federal level, several states have implemented or are creating laws to add more checks to the gabapentin-prescribing process. Ohio, Kentucky, and West Virginia have made it a controlled substance at the state level. In January of 2019 Michigan classified gabapentin as a Schedule 5 substance, which is the same scheduling as cough medicines with codeine. Virginia did the same in July of 2019 and Alabama followed in November of 2019.
Then on December 19, 2019, the FDA released a drug safety communication warning of serious breathing difficulties may occur patients using gabapentinoids with opioids or other drugs that depress the central nervous system, such as anti-anxiety medications, antidepressants, and antihistamines. Individuals with respiratory risk factors that reduce lung function (i.e., COPD: chronic obstructive pulmonary disease) and the elderly are also at higher risk. They are requiring new warnings about the risk of respiratory depression be added to the prescribing information of gabapentinoids.
We have also required the drug manufacturers to conduct clinical trials to further evaluate their abuse potential, particularly in combination with opioids, because misuse and abuse of these products together is increasing, and co-use may increase the risk of respiratory depression. Special attention will be paid to the respiratory depressant effects during this abuse potential evaluation.
There is even less evidence that gabapentin is helpful with mental health disorders. In 2000, a randomized, placebo-controlled trial showed gabapentin did not work better than placebo for bipolar disorder; one study found it was worse than placebos when treating bipolar mania. The British Medical Journal published a study in June of 2019 that found gabapentinoids were associated with increased risk of suicidal behavior and unintentional overdoses in adolescents and young adults (15-24 years old). There were no clear associations with suicidal behavior in those aged 55 and older.
Participants in the other age bands showed heterogeneous associations, with increased hazards of suicidal behaviour, unintentional overdoses, and head/body injuries, and no associations with road traffic incidents or offences and arrests for violent crimes. When analysing gabapentinoids separately, pregabalin use was associated with increased hazards of all outcomes, whereas there were decreased or no associations for gabapentin.
At the Eastern Division StartWell Event 2019 held by the Royal College of Psychiatrists (RC Psych), The President of the RC Psych, Wendy Burn tweeted the following slide from one of the presentations:
Her tweet said: “We are going to have to face the issue of dependence on antidepressants,” but notice that gabapentinoids are also listed. This was a replication of the “Prescribed medicines review: summary” for Gov.UK, which found that 1.4 million people (3% of the adult population of Britain) had received, and had dispensed, a gabapentinoid. This was an increase of 2.9% to 3.3% of the adult population. The number of patients continuously on a gabapentinoid in the UK from April 2015 to March of 2018 was 160,000.
From the time Neurontin (gabapentin) was approved by the FDA in 1994, there have been reports of intentional off-label use, promoted by the pharmaceutical company that brought it to market. Neurontin has been off-patent since 2004, but it seems the off-label use and now misuse of gabapentin has continued, and even grown. It has become a cheap (less than $1 per pill) way to potentiate the high of opioids and is now sold by drug dealers alongside of your opioid-of-choice. It has been even touted as a possible treatment for alcohol dependence. This so-called “cure” for addiction has been turned on its head and been demonstrated to intensify the high from opioids and perpetuate, but not cure, addiction.
I have been monitoring and expressing concern about the use and misuse of gabapentinoids for several years. It seems to me the federal government should take the next step and Schedule gabapentin, particularly now that it has become an adjunct to an opioid high. For more on concerns with gabapentinoids, see “Twentieth Century Snake Oil,” “The Dark Side of a Pill to Cure Addiction,” “Foolishness with Gabapentin,” Risky Alcoholism Treatment,” and “The Evolution of Neurontin Abuse.”