
Kratom is back in the news as the FDA issued a public health advisory related to mounting concerns regarding the risks associated with its use. The FDA Statement from Scott Gottlieb about kratom singled out its use to treat opioid withdrawal as a particular concern. Gottlieb said: “There is no reliable evidence to support the use of kratom as a treatment for opioid use disorder.” Individuals who use kratom are playing doctor, as there are no dependable instructions for its use and there is no consultation with a healthcare professional about the product’s dangers, adverse effects or interactions with other drugs. “There’s clear data on the increasing harms associated with kratom.”
Calls to U.S. poison control centers about kratom increased 10-fold from 2010 to 2015. There are reports of 36 deaths from kratom or kratom-containing products, according to the advisory. Not surprisingly, given the opioid cocktails appearing on the streets, kratom has been reportedly laced with various opioids. “The use of kratom is also associated with serious side effects like seizures, liver damage and withdrawal symptoms.” There are no currently FDA-approved therapeutic uses of kratom.
Before it can be legally marketed for therapeutic uses in the U.S., kratom’s risks and benefits must be evaluated as part of the regulatory process for drugs that Congress has entrusted the FDA with. Moreover, Congress has also established a specific set of review protocols for scheduling decisions concerning substances like kratom. This is especially relevant given the public’s perception that it can be a safe alternative to prescription opioids.
Gottlieb pointed out that kratom is already a controlled substance in 16 countries, “including two of its native countries of origin, Thailand and Malaysia.” Australia, Sweden and Germany are among the other countries listing kratom as a controlled substance. Several states have banned kratom: Alabama, Arkansas, Indiana, Tennessee, Vermont and Wisconsin. Several others, Florida, Georgia, New York, North Carolina and Oregon have pending legislation to ban it. Gottlieb encouraged supporters to conduct the research that will help to better understand kratom’s risk and benefit profile. “In the meantime, based on the weight of the evidence, the FDA will continue to take action on these products in order to protect public health.”
In response to the FDA advisory on kratom, The American Kratom Association (AKA) has asked the FDA to “review and correct” it. It claimed the advisory was based on “discredited, incomplete, and mischaracterized scientific claims” and as a result, it should be rescinded. Medscape reported the AKA has filed a formal dispute resolution petition with the Department of Health and Human Services challenging what it claimed was the “weak scientific basis” of the FDA advisory.
For years, the FDA has published scientifically inaccurate information on the health effects of consuming kratom, directly influencing regulatory actions by the DEA [Drug Enforcement Administration], states, and various local government entities. AKA believes the FDA health advisory on kratom will lead to more state and local bans, all based on discredited, incomplete, and mischaracterized scientific claims.
The AKA disputed the FDA claim that kratom has opioid-like abuse potential, arguing it is primarily used because it is beneficial, and not as a means to get high. The organization also downplays the overdose risk with kratom, saying: “The handful of possible kratom-associated deaths in the US involved people taking multiple drugs, with apparent causes of death varying widely, quite unlike what is seen with narcotic-like opioids.” Reversing the FDA concern that unrestricted kratom use could increase the opioid crisis, the AKA claimed the ban would increase it. The AKA claimed kratom consumers are afraid they will be forced to seek out illegal opioids if kratom was banned. “It would be an outrageous and unacceptable public health outcome if the effect of the FDA assault on kratom backfires and leads to more opioid addiction and death.”
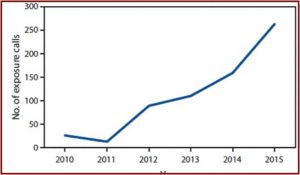
Despite the claims by the AKA, the FDA public health advisory does not seem ill advised. A CDC Morbidity and Mortality Report in 2016 described a study of U.S. poison centers between 2010 and 2015. During the study poison centers received 660 calls about reported exposure to kratom. The number of calls increased tenfold, from 26 in 2010 to 263 in 2015. See the figure below.
 Among the calls, 90.2% reported ingestion of the drug. Isolated exposure, use of kratom alone, was reported by 64.8% of cases. “Among calls reporting use of kratom in combination with other substances (multiple exposures), the most commonly reported other substances were ethanol, other botanicals, benzodiazepines, narcotics, and acetaminophen.”
Among the calls, 90.2% reported ingestion of the drug. Isolated exposure, use of kratom alone, was reported by 64.8% of cases. “Among calls reporting use of kratom in combination with other substances (multiple exposures), the most commonly reported other substances were ethanol, other botanicals, benzodiazepines, narcotics, and acetaminophen.”
Medical outcomes associated with kratom exposure were reported as minor (minimal signs or symptoms, which resolved rapidly with no residual disability) for 162 (24.5%) exposures, moderate (non-life threatening, with no residual disability, but requiring some form of treatment) for 275 (41.7%) exposures, and major (life-threatening signs or symptoms, with some residual disability) for 49 (7.4%) exposures.
There was one death reported in the CDC Report, but the person had also ingested paroxetine (Paxil) and lamotrigine (Lamictal) in addition to the kratom. While the FDA advisory said it was aware of 36 deaths associated with kratom products, it did not provide further information. The August 2016 DEA announcement to schedule kratom, which was later rescinded, said the DEA was aware of 15 kratom-related deaths between 2014 and 2016; but again did not provide any further information.
An article in BioMed Research International, “Following ‘the Roots’ of Kratom” described several short term adverse effects from kratom, including nausea, constipation, sleep problems, itching, sweating, erectile dysfunction. Long-term adverse effects include: anorexia, dry mouth, darker skin and hair loss. Withdrawal symptoms can include: hostility, aggression, muscle and bone aches, jerky limb movements, anorexia and weight loss, and insomnia. Kratom could be a deadly substance when mixed with other compounds. Multiple fatalities with a kratom product known as “Krypton” have been reported. See “Following ‘the Roots’ of Kratom,” for more particulars on the reported deaths. For more information on Krypton, see “Krypton Can Kill You.”
Table 1 of the article listed several substances found mixed with kratom in fatalities, including antidepressants, a mood stabilizer and a hypnotic sleep aide: O-desmethyltramadol (a metabolite of tramadol); propylhexedrine (an analog of methampheamine); over-the-counter cold medications and benzodiazepines; venlafaxine (Effexor), diphenhydramine (Benadryl), and mirtazapine (Remeron); zopiclone (Zopiclone), citalopram (Celexa), and lamotrigine (Lamictal).
According to preclinical data and case reports published in scientific literature as well as anecdotal experiences posted online, kratom is not a safe drug. Its consumption is associated per se with drug dependency, development of withdrawal symptoms, craving, serious adverse effects, and life-threatening effects, especially in a multidrug-intoxicating scenario.
Another article in the International Journal of Legal Medicine, “The Pharmacology and Toxicology of Kratom,” said there was growing international concern for the kratom’s effects and safety due to “an increase in hospital visits and deaths in several countries that are said to have been caused by extracts of the plant.” The abuse potential of kratom “requires careful evaluation of its benefits and potential toxicities.”
So what is next with kratom? When the DEA reversed its decision to temporarily classify kratom as a Schedule I Controlled Substance in October of 2016, it said it would solicit public comments (which it did) and review the FDA’s “scientific and medical evaluation” of the proposed scheduling. The FDA public health advisory for kratom indicates the agency concluded there was cause for concern in keeping it unregulated. I’d guess that further action by the DEA to schedule kratom will be delayed, pending the outcome of the AKA’s suit against the FDA.
Gottlieb’s public encouragement of research into kratom’s possible use as a therapy for “a range of disorders” may suggest room for a scheduling of kratom other than as a Schedule I Controlled Substance. If kratom were to be placed even temporarily as a Schedule I controlled Substance, further research into its potential medical benefits would be difficult to conduct. Funding for kratom research is also hard to come by. Obtaining kratom of the quality needed for research is difficult as well. A researcher referred to the FDA requirements to develop a clinical trial for kratom as a “bureaucratic nightmare.” Edward Boyle, a kratom researcher, said: “Is it an effective treatment for opioid withdrawal, or is it another pathway to addiction? I don’t think anybody has a defined concept of where it actually lies on that continuum.”
See “The Secret of Kratom” and “Kratom: Part of the Problem or a Solution?” for more information on kratom.





