Antidepressant Fall From Grace, Part 1
The so-called antidepressants are not a single class of drugs; nor are they just used to treat depression. There has also been a long-running debate over their adverse effects and treatment effectiveness. After Prozac was approved as the first SSRI (selective serotonin reuptake inhibitor) in 1987, the SSRI class of antidepressants became a kind of patent medicine for treating various mood-related conditions, and was even used as a character or personality enhancement. Yet there has been an accumulation of evidence over the past twenty years that questioned whether SSRIs were more effective than placebo. Are antidepressants effective treatments for depression and are they worth the risk?
Currently, the main classes of antidepressants are SSRIs such as Prozac (fluoxetine), Zoloft (sertaline) and Celexa (citalopram); SNRIs (serotonin norepinephine reuptake inhibitors such as Effexor (venafaxine), Cymbalta (duloxetine) and Pristiq (desvenlafaxine); and NDRIs (norepinephrine-dopamine reuptake inhibitors) such as Welbutrin or Zyban (bupropion). Methylphenidate (as Ritalin, Concerta and others) is also chemically a NDRI, but is used primarily as a medication for ADHD and will not be included in the following discussion. Older classes of antidepressants include tricyclic antidepressants (TCAs), monoamine oxidase inhibitors (MAOIs) and tetracyclic antidepressants (TeCAs). Antidepressants are used to treat major depression, anxiety, obsessive-compulsive disorder (OCD), attention-deficit hyperactivity disorder (ADHD), eating disorders, chronic and neuropathic pain, bed-wetting, fibromyalgia and menopause, smoking cessation and others.
The earliest and probably most widely accepted scientific theory of antidepressant action is the monoamine hypothesis (which can be traced back to the 1950s), which states that depression is due to an imbalance (most often a deficiency) of the monoamine neurotransmitters (namely serotonin, norepinephrine and dopamine).It was originally proposed based on the observation that certain hydrazine anti-tuberculosis agents produce antidepressant effects, which was later linked to their inhibitory effects on monoamine oxidase, the enzyme that catalyses the breakdown of the monoamine neurotransmitters. All currently marketed antidepressants have the monoamine hypothesis as their theoretical basis.
In 1952 psychiatrists Max Lurie and Harry Salzer coined the term “antidepressant” to describe the action of isoniazid, a medication originally developed as a treatment for tuberculosis. Seeikoff and Robitzek experimented with another ant-tuberculosis drug, iproniazid, which had a greater psychostimulant effect, but also greater toxicity. Serious adverse effects, including liver inflammation, led to its recall as an antidepressant in 1961. These drugs are MAOIs.
A tricyclic antidepressant, Tofranil (imipramine), was also first used to treat depression in the 1950s. Another TCA, Elavil (amitriptyline), was approved in 1961. Dozens of additional TCAs were developed over time. Similar to TCAs, tetracyclic antidepressants (TeCAs) like Remeron (mirtazapine) were introduced in the 1970s. But there were problems with TCAs, including a higher risk of serious cardiovascular side effects. They also had a relatively low toxicity level, making them a suicide risk—not an attractive adverse effect for an antidepressant.
While they are biochemically similar to TCAs and have no real differences in therapeutic effectiveness, SSRIs only affect the reuptake of serotonin, not the reuptake of dopamine and norepinephrine. SSRIs also have a higher toxicity level than TCAs and a lower risk of serious cardiovascular side effects. So an argument was made for SSRIs having fewer and milder side effects than TCAs. Initially persuasive, this claim has become less credible over time.
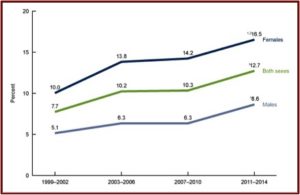
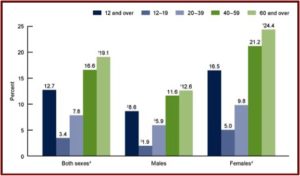
The SSRI antidepressant craze began with the introduction of Prozac in 1987. Zoloft (sertaline) came to market in 1992, Luvox (fluvoxamine) in 1994, Paxil (paroxetine) in 1996, Celexa (citalopram) in 1998, and Lexapro (escitalopram) in 2002. All the above SSRIs are now off patent and available as generics. Yet they are among the three most commonly used classes of prescription medications in the U.S. 12.7% of persons over the age of 12 reported they took an antidepressant in the previous month, according to data from the National Center for Health Statistics. Antidepressant use is highest among females in two age groups: 40 to 59 (21.2%), and 60 and over (24.4%). The same trend was seen with males from 40 to 59 (11.6%), 60 and over (12.6%). See the linked CDC article for more information on antidepressant use among Americans.
Prozac use swept over the U.S. like a pharmaceutical wave after it was approved. It even became a drug that people took for “cosmetic psychopharmacology,” according to psychiatrist Peter Kramer, the author of the best-selling book: Listening to Prozac. Kramer said: “If I am right, we are entering an era in which medication can be used to enhance the functioning of the normal mind. The complexities of that era await us.”
The complexities of antidepressant use from the early days included evidence of violence and suicide. Toxic Psychiatry by another psychiatrist named Peter Breggin, was published in 1991. Breggin documented reports of suicidal behavior with Prozac in both the popular press and the professional literature. “Suicidal Behavior Tied to Drug,” was published on February 7, 1991 in The New York Times. The article said two cases of suicidal behavior and fantasies (with no prior history) were reported in The New England Journal of Medicine that same day. Eli Lilly (the manufacturer of Prozac) was facing more than 50 lawsuits at the time, but denied that there was any scientific merit to the claim Prozac could prompt suicidal or violent acts.
Dr. Breggin also predicted the rise of what is now called “treatment resistant depression” with SSRIs. He said: “If Prozac can indeed alleviate depression by making more serotonin available in the brain, then with time it may produce incurable depression by making the brain relatively unresponsive to any amount of serotonin.” In 2004 the FDA finally required black box warnings to be placed on the newer antidepressants, warning of the potential for the increased risk of suicidal thoughts and behavior in children and adolescents. Despite the age qualification, the danger for adults is also present.
In an article, Breggin described “How FDA Avoided Finding Adult Antidepressant Suicidality.” Quoting the FDA report of the 2006 hearings, he noted where the FDA permitted the drug companies to search their own data for “various suicide-related text strings.” Because of the large number of subjects in the adult analysis, the FDA did not—repeat, DID NOT—oversee or otherwise verify the process. “This is in contrast to the pediatric suicidality analysis in which the FDA was actively involved in the adjudication.” He added that the FDA did not require a uniform method of analysis by each drug company and an independent evaluator as required with the pediatric sample.
Peter Gøtzsche, a Danish physician and medical researcher who co-founded the Cochrane Collaboration, wrote an article describing how “Antidepressants Increase the Risk of Suicide and Violence at All Ages.” He said that while drug companies warn that antidepressants can increase the risk of suicide in children and adolescents, it is more difficult to know what that risk is for adults. This is because there has been repeated underreporting and even fraud with reporting suicides, suicide attempts and suicidal thoughts in placebo-controlled antidepressant trials. He added the FDA has contributed to the problem by downplaying the concerns, choosing to trust the drug companies and suppressing important information.
Gøtzsche drew attention to a meta-analysis of placebo-controlled trials from 2006 where the FDA reported five suicides in 52,960 patients (one per 10,000). See Table 9 of the 2006 report. However the individual responsible for the FDA’s 2006 meta-analysis had published a paper five years earlier using FDA data where he reported 22 suicides in 22,062 patients (which is 10 per 10,000). Additionally, Gøtzsche found there were four times as many suicides on antidepressants as on placebo in a 2001 study.
Additional adverse side effects from antidepressant use include: weight gain and metabolic disturbances; sexual dysfunction; bleeding; sleep disturbances; emotional blunting; agitation and activation; discontinuation syndrome (withdrawal); violence; and others. New research published in the journal Psychotherapeutics and Psychosomatics concluded that SNRIs should be added to the list of drugs that induce withdrawal symptoms upon discontinuation. Even a gradual withdrawal did not prevent the onset of “withdrawal phenomena” with SNRIs.
The results of this systematic review indicate that withdrawal symptoms may occur after discontinuation of any type of SNRI (venlafaxine, desvenlafaxine, duloxetine, milnacipran, or levomilnacipran). However, the prevalence of withdrawal symptoms was variable and appeared to be higher after discontinuation of venlafaxine.
See a literature review of long-term use of newer generation antidepressants (i.e., SSRIs and SNRIs and others) by Carvalho et al. You can also look at “In the Dark About Antidepressants,” Antidepressant Misuse Disorder” and “Listening to Antidepressants” on this website for more information on antidepressants and their adverse effects. For more information on the association of antidepressants and violence, see Medication Madness by Peter Breggin and “Violence and the Brain” or “Iatrogenic Gun Violence” on this website.
While not everyone will experience these adverse events, they are present for many individuals who have used or are using antidepressants. But if your depression is debilitating, are antidepressants effective enough to be worth risking their potential adverse effects? In Part 2 of “Antidepressant Fall From Grace” we will look at the debate over the efficacy of antidepressants.