A Shot Across the Bow of Pharma
Ending a four year-long federal investigation, Avanir Pharmaceuticals agreed to pay an estimated $116 million in criminal penalties and civil damages in a settlement reached with the Department of Justice (DOJ) on September 26, 2019. According to FiercePharma, the company also agreed to assist in the prosecution against former employees and a top prescriber of its bestselling drug, Nuedexta. As a part of the deal, Avanir agreed to a five-year Corporate Integrity Agreement with the Department of Health and Human Services (HHS). Avanir CEO Wa’el Hashad said the “company takes its responsibilities to patients, their families and caregivers, and healthcare providers very seriously. . . . Avanir is deeply committed to regulatory and legal compliance, integrity and ethical behavior, and the health and safety of patients.”
The DOJ reported Avanir agreed to pay over $95 million to resolve civil False Claims Act allegations that the company paid kickbacks to a physician to prescribe Nuedexta, and that it made false and misleading marketing claims about Nuedexta to long-term care facilities. Allegedly the false claims were targeted to influence providers to prescribe Nuedexta as an alternative to antipsychotic drugs. The federal government was trying to limit the use of antipsychotics as “chemical restraints” used to manage behaviors commonly associated with dementia patients.
Avanir has agreed to pay $95,972,017 to the United States to resolve allegations under the False Claims Act related to its marketing of Nuedexta. The government alleged that between October 29, 2010, and December 31, 2016, Avanir provided remuneration in the form of money, honoraria, travel, and food to certain physicians and other health care professionals to induce them to write prescriptions for Nuedexta. One form of remuneration included Avanir’s payment to certain health care professionals to give talks (commonly known as “speaker’s programs”) about Nuedexta based on their willingness to prescribe Nuedexta. These events were primarily social, with no educational value.
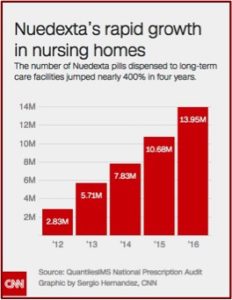
Nuedexta is only approved as a treatment for a rare condition called pseudobulbar affect (PBA) that causes uncontrollable laughing or crying that is not connected to a person’s mood. PBA is extremely rare in dementia patients, affecting less than 5%. It is most commonly associated with people who have multiple sclerosis (MS) or Lou Gehrig’s disease, ALS. Doctors were found to be inappropriately diagnosing PBA to justify using Nuedexta to treat difficult to manage elderly patients. There was a CNN investigation in 2017 that indicated between 2012 and 2016 Nuedexta sales jumped 400%; more than half of which had gone to long-term care facilities. For more on this, see: “Conjuring Diagnoses For the Elderly.”
According to a recent CNN report, whistleblowers alleged that from the drug’s early years Avanir illegally directed salespeople to market Nuedexta in nursing homes as an alternative to antipsychotic drugs specifically for “use in controlling the behavior of patients prone to disruptive outbursts.” They also claimed salespeople coached doctors on how to describe patients’ conditions in order to guarantee approval, and even forged physician signatures on paperwork for insurers. One lawsuit stated, “At least one Avanir (salesperson) went so far as to dress in scrubs, review patients’ files at the nurses’ station in nursing homes, and write the diagnosis for PBA in the medical files of patients.” These tactics were allegedly praised by an executive on a national sales call. Government data indicated Medicare Part D spent around $225 million on Nuedexta in 2017, a 700% increase since 2012.
Assistant Attorney Jody Hunt of the DOJ said kickbacks can corrupt a provider’s medical judgment. “And it is particularly concerning when a pharmaceutical company uses kickbacks to drive up sales in connection with a vulnerable population, such as elderly patients in nursing care facilities.” The government alleged Avanir sought to take advantage of efforts by the Centers for Medicare and Medicaid Services to reduce the use of antipsychotics with dementia patients. Avanir instructed its sales force to initiate discussions in long-term care (LTC) facilities about antipsychotic use and how Nuedexta could be used to reduce their reliance on antipsychotics.
Avanir’s own studies demonstrated that the actual population of patients with PBA is limited. In order to counter the objection by certain physicians that they had few, if any, patients that exhibited signs of PBA in their facilities, Avanir instructed sales representatives to provide false and misleading information that PBA patients could be exhibiting a wide variety of “behaviors” such as crying without tears, moaning, or making other inarticulate sounds, when, in fact, those symptoms are commonly observed in patients who have dementia but do not have a diagnosis of PBA. This strategy worked, and Nuedexta utilization in LTC facilities increased.
The DOJ reported the civil settlement resolved lawsuits by three whistleblowers, who were all former employees of Avanir. Under the whistleblower provisions of the False Claims Act, private citizens are permitted to sue on behalf of the government for false claims and to share in any recovery.
The Employment Law Group® law firm reported Kevin Manieri was the first person to report the company’s activities to authorities. A biopharmaceuticals executive with decades of experience, he was fired only months after joining Avanir in 2014 when he complained to an Avanir vice president about the company using “speaker fees” to reward doctors for writing unnecessary prescriptions for Nuedexta. Many of Avanir’s speaking engagements were small, sparsely attended gatherings at local restaurants. The speakers tended to be individuals willing to diagnose PBA “based upon a bare minimum of symptoms,” according to Kevin Manieri’s complaint. A press release issued by the U.S. Attorney’s Office for the northern District of Ohio said many of the Nuedexta speaking engagements had “little to no educational value.”
According to the complaint filed by Mr. Manieri, whom the drug company hired to oversee Nuedexta sales to physicians in the northern U.S., Avanir pushed its reps to focus on a few high-volume prescribers who were willing to recommend Nuedexta to patients who likely didn’t need the drug. He cited a vivid example in the complaint: A Cleveland-area neurologist who wrote twice as many Nuedexta prescriptions as any other doctor in the U.S. — and who also was Avanir’s highest-paid speaker, with 42 engagements in 12 months yielding more than $56,000 in payments.
Sadly, Avanir’s marketing strategy is not unusual in the world of pharmaceutical companies. According to Dr. Marcia Angell in The Truth About Drug Companies, Parke-Davis paid academic experts to put their names on flimsy research papers that supposedly showed Neurontin (gabapentin) was effective for certain off-label conditions. The result was Neurontin becoming a blockbuster drug, with over 2 billion in sales for 2003. “About 80 percent of prescriptions that year were for unapproved uses—conditions like bipolar disorder, post-traumatic stress disorder, insomnia, restless legs syndrome, hot flashes, migraines, and tension headaches.” In fact, Neurontin became an all-purpose restorative for chronic discomfort. An internal company e-mail described Neurontin as “the ‘snake oil’ of the twentieth century.”
In May of 2004, Warner-Lambert, of which Parke-Davis was then a division, agreed to pay $430 million to resolve criminal charges and civil liabilities related to its “illegal and fraudulent promotion of unapproved uses” for Neurontin. See the Department of Justice announcement here. Also see “Twentieth Century Snake Oil” for more on this.
The larger issue is that we can no longer trust much of the clinical research that is published. Among the concerns are reportedly selective publication of clinical trials, rigging the outcomes of those trials, publication bias and industry payments to medical journals and their editors. Richard Horton, and editor in chief of The Lancet said: “The case against science is straightforward: much of the scientific literature, perhaps half, may simply be untrue.” Marcia Angell, a former editor in chief of the New England Medical Journal (NEJM) said: “It is simply no longer possible to believe much of the clinical research that is published, or to rely on the judgment of trusted physicians or authoritative medical guidelines. I take no pleasure in this conclusion, which I reached slowly and reluctantly over my two decades as an editor.” See “Corrupted Clinical Trials” for more on this.
So, the actions of whistle blowers like Kevin Manieri are not simply the acts of disgruntled ex-pharmaceutical employees in the long run. What happened to a relatively small pharmaceutical company, Avanir, and its attempts to expand the marketing reach of its featured drug, Nuedexta, are hopefully a warning shot across the bow of Pharma. Marketing rhetoric disguised as “treatment” or “evidence-based medicine” will not be tolerated. And there will be individuals and firms like The Employment Law Group® ready to hold you accountable for your actions. For more on Avanir and Nuedexta, also see “A Reason to Cry Uncontrollably.”