Back to the Future with Psychedelics
“I am 100 percent in favor of the intelligent use of drugs, and 1,000 percent against the thoughtless use of them, whether caffeine or LSD.” (Timothy Leary, in Chaos and Cyber Culture)
We’re going “back to the future” with recent research into the therapeutic benefits of hallucinogens for treating alcoholism and mood disorders. (See additional stories here and here; and a previous blog, “As Harmless as Aspirin?”) Classical hallucinogens such as LSD, mescaline or psilocybin, and dissociative anesthetics such as ketamine and PCP might be “useful” in the treatment of major depression, anxiety disorders and OCD. A recent study concluded: “There was evidence for a beneficial effect of LSD on alcohol misuse.” A single dose of LSD was found to be associated with a decrease in alcohol misuse. Another longitudinal study suggested that: “hallucinogens may promote alcohol and drug abstinence and prosocial behavior in a population with high rates of recidivism [with individuals on probation or parole].”
An issue of Current Drug Abuse Reviews (volume 6, number 1, 2013) was devoted to the investigation of psychedelics and their potential as therapeutic agents in the treatment of addiction. Several different articles suggested the therapeutic benefits of a variety of psychoactive substances—some classics and some newer ones.
Rick Doblin, in “Psychedelic-Assisted Psychotherapy for the Treatment of Addiction,” said: “There are multiple frameworks for understanding how psychedelic therapy can alleviate substance abuse.” He noted that the idea that psychedelics can be helpful in combating drug abuse conflicts with “the notion that psychedelic drug use is inherently wrong.”
Michael Bogenschutz of the University of New Mexico Health Sciences Center suggested that sacramental use of classic hallucinogens, like the Native American Church’s use of peyote, “is strongly associated with decreased alcohol and drug use.”
Lisa Jerome and others lobbied for studies that tested MDMA-assisted psychotherapy in people with an active substance use disorder. “It appears that MDMA, like classic psychedelics, may have a place in addressing substance abuse or dependence, which could be linked to its pharmacology or its psychological effects.”
Ayahuasca, a psychotropic brew prepared from an Amazonian vine and bush, may be associated with reduced substance use and “improvements in several cognitive and behavioral states.”
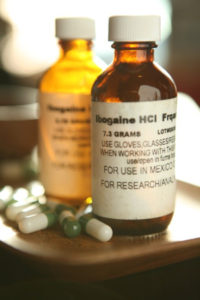
Thomas Kingsley Brown reported that ibogaine, a psychoactive alkaloid found in a rainforest shrub of West Central Africa, helps with withdrawal symptoms and reduces drug cravings.
A study of ayahuasca-assisted treatment for substance use problems by Gerald Thomas and others suggested that it was associated with significant improvements in several factors related to problematic substance use. While this particular study occurred in Canada, ayahuasca has been used as a remedy to help overcome drug addictions in Peru and Brazil. “Although these programs claim improved health outcomes for patients who complete them, neither has been evaluated with sufficient scientific rigor to provide definitive evidence of the success of their approaches.”
Ibogaine is not used in the US to treat addiction because of its severe side effects, which include hallucinations, bradycardia (slow heart rate), whole-body tremors and ataxia (lack of muscle control during voluntary movements). It also had cerebellar toxicity with high doses in rats. Nevertheless, it is a growing form of treatment outside the US. A subculture of ibogaine clinics has sprung up in Mexico. Read about a trip to one here.
A synthetic derivative of ibogaine, 18-MC, has been developed and is said to show promise. It resulted in “a long-lasting decrease in ethanol, morphine, cocaine, methamphetamine and nicotine self-administration [in rats], and attenuation [decrease] of opioid withdrawal symptoms.” Significantly, it is not expected to have hallucinogenic effects and does not have the negative side effects noted above with ibogaine.
In 2012 Savant HWP, a privately-owned pharmaceutical company in California, received a three-year grant from the National Institute on Drug Abuse (NIDA) for the pre-clinical development of 18-MC. Stanley Glick, the scientific founder of Savant and a long time researcher with ibogaine, said: “18-MC is likely to be the first of a new generation of agents effective against a broad spectrum of addictions—from hard drugs such as heroin and cocaine, to alcohol, nicotine and even sugary, high-fat foods, possibly reducing obesity rates.” On September 23rd of 2014 Savant announced they had begun human safety clinical trials on 18-MC. “Savant HWP plans to develop 18-MC as a treatment for many forms of addiction and compulsive behavior, with an initial focus on cocaine and opiate dependencies.”
The so-called “psychedelic treatment” approach, based on the original work of Humpry Osmond, uses pre and post therapeutic sessions and one large dose of your hallucinogen-of-choice (LSD, ayahuasca, psilocybin, mescaline). The spiritual, therapeutic goal is captured here by Aldous Huxley’s description of his experience with mescaline in The Doors of Perception:
The man who comes back through the Door in the Wall will never be quite the same as the man who went out. He will be wiser but less cocksure, happier but less self-satisfied, humbler in acknowledging his ignorance yet better equipped to understand the relationship of words to things, of systematic reasoning to the unfathomable Mystery which it tries, forever vainly, to comprehend.
But we should also remember the warnings of Albert Hofmann, the inventor of LSD, who cautioned not to underestimate the potential negative consequences of a deliberate provocation of mystical experiences with hallucinogens like LSD. “Wrong and inappropriate use has caused LSD to become my problem child.” In the “LSD state” the boundaries between the self and the outer world effectively disappear. “A portion of the self overflows into the outer world. . . . This can be perceived as a bless[ing], or as a demonic transformation imbued with terror.”
Originally posted on December 22, 2014.