The Truth About Gabapentin
Gabapentin has been peddled as a treatment for problems as diverse as hot flashes, itchy skin, postoperative pain, and social phobia. That is in addition to its approved uses for partial seizures, nerve pain following shingles and moderate-to-severe restless leg syndrome. In November of 2021, GoodRx listed gabapentin as the sixth-most prescribed medication in the U.S. Not surprisingly, in a 1999 email the Marketing Director for Pfizer at the time called gabapentin “the snake oil of the twentieth century,” claiming it had been successful with “just about everything they have studied.”
Pfizer has paid $945 million to resolve lawsuits against the off-label marketing of gabapentin. For a number of years, gabapentin has been known as a drug of abuse—alone or in combination with other drugs. A 2017 study in the journal Addiction by Lyndon et al (discussed below) noted combining gabapentin (Neurontin) or pregabalin (Lyrica) with opioids increased the risk of acute overdose death. The researchers referred to concomitant use of gabapentinoids and heroin as an emerging public health problem.
It is important that doctors and their patients are aware that the combination of opioids with gabapentin or pregabalin potentially increases the risk of acute overdose death through either reversing tolerance or by an additive effect of the drugs to depress respiration.
In 2019 the FDA acknowledged the risk of serious breathing problems with gabapentin and pregabalin and added a warning to their prescribing information. The FDA also required manufacturers to conduct clinical trials to further evaluate their abuse potential, particularly in combination with opioids. “Misuse and abuse of these products together is increasing, and co-use may increase the risk of respiratory depression.” Despite these concerns, gabapentin is still not a Scheduled drug by the DEA, while pregabalin, coming to market in 2004, is a Schedule V controlled substance.
However, Campbell et al reported in “Gabapentin controlled substance status” that seven state boards of pharmacy have independently reclassified gabapentin as a Schedule V drug. Twelve states have implemented prescription monitoring programs and three states are deliberating gabapentin’s future controlled/monitored status. The United Kingdom reclassified gabapentin in 2019, placing it in the same controlled substance schedule as barbiturates, buprenorphine, and tramadol. Despite the lawsuits and FDA-limitations, gabapentin prescriptions dispensed from 2011 to 2017 increased two-fold—from 33.4 million to 64.8 million.
Case reports indicate that gabapentin is abused for a variety of reasons, but the most common listed are for euphoria, potentiating the high from opiates, reduction of alcohol cravings, a cocaine-like high, as well and sedation or sleep. Individuals at the highest risk for abusing gabapentin include those with opioid abuse, mental illness, or previous history of prescription drug abuse. Postmortem toxicology analyses have directly linked gabapentin as a cause of death, but most deaths observed occurred with concomitant use with opiates or benzodiazepines. Gabapentin when combined with these agents appears to be lethal at lower serum gabapentin concentrations than gabapentin alone.
Medical Xpress confirmed that most prescriptions for gabapentin were approved for off-label use. A newly published study, “Outpatient Off-Label Gabapentin Use for Psychiatric Indications,” found that more than 99% were for off-label uses. Amie Goodin, a coauthor of the study, said they had anticipated a lot of off-label use, but were surprised at the magnitude of use.
Out of more than 200,000 patient records, just over 5,700 involved a gabapentin prescription. That corresponds to nearly 130 million visits nationally between 2011 and 2016.The vast majority of those prescriptions were off-label, and most patients were also on other prescription drugs. In nearly one-third of cases, those additional medications included a CNS depressant. Antidepressants were the most common type of CNS depressant, followed by opioid painkillers and benzodiazepines. Of all office visits where off-label gabapentin was in the record, about 5% of patients had a depression diagnosis, and 3.5% had an anxiety disorder.
On March 7, 2022, Medscape announced that Public Citizen filed a petition with the FDA and the DEA to make gabapentin a federally controlled substance. The nonprofit group requested that gabapentin come under the DEA’s Schedule V category, where Lyrica is scheduled. The authors argued the risks with gabapentin warranted more safety measures. Classifying gabapentin as a Schedule V drug would make tracking its use and misuse easier and put into place educational and limitation requirements to lessen the risks of addiction, overdose and death.
The Public Citizen petition said there was substantial evidence of widespread misuse of gabapentin, partly because of the extraordinary levels of off-label prescribing. “Both gabapentin and pregabalin have been empirically linked to the opioid overdose epidemic as drugs that potentiate the activity of these oftentimes deadly analgesics.” There were five systematic reviews summarizing evidence of the harms associated with gabapentin abuse, misuse and diversion.
One review by Smith et al, “Gabapentin misuse, abuse, and diversion: A systematic review,” concluded from their review that gabapentin was “primarily misused for recreational purposes, self-medication, or intentional self-harm, and was used alone or in combination with other substances, especially opioids, benzodiazepines, or alcohol.”
In summary, findings from the present review suggest that gabapentin is misused/abused internationally for recreation, self-medication, or self-harm, with an array of subjective experiences. Substance abuse populations, especially individuals with a history of or current opioid misuse, appear to be at particular risk for misuse/abuse. Further studies to identify risk factors for gabapentin misuse and to characterize gabapentin’s abuse liability are recommended.
Another systematic review by Evoy et al in 2021, “Abuse and Misuse of Pregabalin and Gabapentin: A Systematic Review Update,” confirmed the findings of their 2017 systematic review, that gabapentin and pregabalin are increasingly being misused in order to self-medicate and produce rewarding effects, such as euphoria, relaxation, or disassociation. “Most concerning was the finding of increased evidence of associated patient harm, including increased hospital utilization and opioid-related overdose risk.”
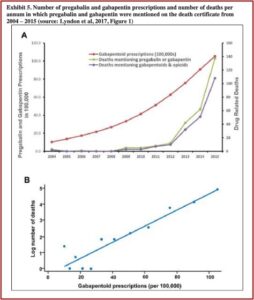
Lyndon et al examined trends in drug-related deaths involving gabapentin in England and Wales from 2004 to 2015 in, “Risk to heroin users of poly-drug use of pregabalin or gabapentin.” They found that prescriptions for gabapentin and pregabalin increased around 24% per year during the study period, from 1 million in 2004 to 10.5 million in 2015. There were concurrent increases in drug-related deaths involving gabapentin and pregabalin; 79% of which involved opioids. “For each increase of 100,000 gabapentinoid prescriptions, the number of deaths increased by approximately 5%.” See the following graphs in the Public Citizen petition.
Postmortem studies in some U.S. jurisdictions have noted an increase in gabapentin-related overdoses. In “Prevalence of gabapentin in drug overdose postmortem toxicology testing results,” Slavova et al found 22% of decedents tested positive for gabapentin. “Among the 3,360 drug-overdose decedents who tested positive for opioids, 880 (26%) also tested positive for gabapentin. Conversely, among the 931 decedents who tested positive for gabapentin, 876 (94%) also tested positive for opioids.”
Buttram et al found in “Law Enforcement-derived data on gabapentin diversion and misuse, 2002-2015” that there were 407 cases of gabapentin diversion reported in 41 states from 2002 to 2015. The gabapentin diversion rate rose steadily from zero in the first quarter of 2002 to a rate comparable to the diversion rate of OxyContin in 2015.
The Public Citizen petition concluded by noting the evidence presented in the petition clearly fulfilled the DEA criteria for scheduling gabapentin:
- There is evidence that individuals are taking gabapentin in amounts sufficient to create a hazard to their health and to the community.
- There is significant diversion of gabapentin from legitimate drug channels.
- Individuals are taking gabapentin on their own initiative rather than on the basis of medical advice from a practitioner.
I’m hopeful the FDA and the DEA will respond to the petition by scheduling gabapentin. I’ve been disturbed with what I’ve seen happen with gabapentin since I first read The Truth About Drug Companies. Marsh Angell described how Neurontin (gabapentin) was transformed from an add-on medication when other anti-seizure drugs failed, into a blockbuster, with sales of $2.7 billion in 2003 by getting doctors to prescribe it for unapproved uses. About 80% of the prescriptions that year were for off-label use. “In fact, Neurontin has become a sort of all-purpose restorative for chronic discomfort of almost any type—yet there is almost no good published evidence that it works for most of these conditions.”
For more information on gabapentin and pregabalin, see “Twentieth Century Snake Oil,” “The Evolution of Neurontin Abuse,” “Foolishness with Gabapentin,” “Gabapentinoids Perpetuate Addiction” on this website.