Brain Stimulation or Brain Damage? Part 2
On Valentine’s Day 1974, British doctors destroyed sections of Derek Hutchinson’s hypothalamus without his consent. Surgeons drilled two holes into his forehead and then sent a wire with an electrical tip deep into his brain. Before the right and left sides of the targeted area of his hypothalamus was burned for thirty seconds each, the surgeon test-stimulated his brain with five to ten volts of electricity to see whether the targeted area of his brain actually controlled emotions, and not pathways crucial to motor functions like walking, talking and breathing. They asked him if he was frightened or angry and he yelled, “Stop it, or I’ll kill you.” Then they proceeded to destroy the targeted areas of his hypothalmus.
As Danielle Egan related in her article, Derek had an hypothalomotomy, a psychosurgery procedure first developed in the 1950s to curb aggressive behavior. Over the next two decades he had repeated overdoses and hospital stays during which he received ECT. At some point, he attacked one of his psychiatrists, but no charges were filed against him. After his wife gave birth to twins, he threatened her with a gun. He met his current wife in a pub and they were married in 1982. He’s struggled with bouts of depression, impotence, insomnia, extreme fatigue and rage since the surgery. “He’s had eight heart attacks, two strokes, has battled anorexia (the hypothalamus also regulates hunger, blood pressure and hormones) and has tried to kill himself seven times.”
An MRI revealed extensive damage to his hypothalamus. They told him “Half of your hypothalamus is gone.” He said that was when he finally woke up. These days Hutchinson is an activist against the use of psychosurgeries and deep brain stimulation (DBS). He thinks psychosurgery should be banned outright and believes mental disorders are caused by an individual’s unique life experiences, and are not a product of a dysfunctional brain.
We must put an end to this immoral, unethical activity being conducted in the name of mental health research… These doctors are guinea-pigging innocent people. These surgeries should never have been invented. They kill the person and they don’t even know they’re not the same person anymore.
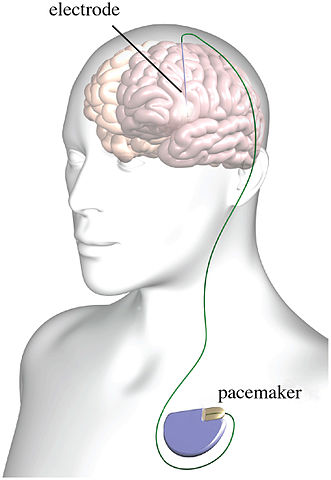
There are clear differences between the hypothalomotomy done to Derek Hutchinson and DBS, which seeks to electrically stimulate specific areas of the brain, not destroy them. However, the adverse effects are similar. In another article for Mad in America on DBS, Egan noted that numerous studies have documented serious adverse mood behavior and personality effects. “These include suicide, depression, apathy, fatigue, mania and serious impulse control issues, such as hypomania, aggression, addiction (to gambling, shopping, drugs, alcohol) and hypersexuality, sometimes resulting in criminal behaviour, including pedophilia.” See “Deep Brain Problems” for more on the adverse effects with DBS.
Andrew Scull described the results of two double-blind studies of DBS for depression. The first one by Dougherty et al was prompted by encouraging response rates in multiple open-label DBS trials. But there was not a significant difference in response rates between the active and control groups. “Our results … failed to demonstrate a significant difference between the active and sham-controlled groups during the blinded phase of the study.” Even worse, adverse events were more frequent in the active group than in the control group for worsening depression, insomnia, irritability, suicidal ideation, hypomania, disinhibition and mania.
There was even one completed suicide in the active treatment group. However, it occurred after the person stopped treatment because of a failure to improve and was awaiting removal of the electrodes. “The authors decided that that adverse event did not count!” Notice that the second listed author of this study, Ali Rezai, would later emerge as the lead investigator for the first U.S. clinical trial of DBS for heroin described in Part 1 of this article.
The second trial was conducted by Helen Mayberg and Andres Lozano, who are both well known supporters of DBS, and a couple dozen others. Again, there was no statistically significant difference between the stimulation group and the control group. Scull reported the researchers anticipated those receiving active stimulation would improve twice as much. Both the treatment and the placebo groups improved, but the improvement was slight and not statistically significant.
Thus, once put to a controlled test, the claims for deep brain stimulation as a treatment for depression resoundingly failed. The purely speculative and fanciful biological “theory” of depression on which the intervention rested had not produced the anticipated results.
Then in October of 2019, Mayberg published another DBS study, claiming the procedure was generally safe and well tolerated. “The rate of medical or surgical complications was consistent with the rate observed in studies of DBS for other indications.” Oh, and there were no completed suicides. Yet Andrew Scull pointed out five of the small sample of 28 individuals dropped out after 1, 2, 5, 8 and 11 years. There were 56 adverse events. One patient experienced ten of them and had the electrodes removed after two years and dropped out of the study.
What are we talking about here? Nineteen of the events involved the surgery going wrong in a variety of major ways. Six infections resulted from the brain surgery. Six patients had to have the original device “explanted,” as the authors put it, because the wires caused an infection (3 cases), failed to work (2 cases), or the crude targeting of the device needed adjusting (1 case). Another patient experienced hemorrhage of the cortex and a post-operative seizure. The device failed in 15 cases. There is no further discussion of these iatrogenic disasters, or the suffering they entailed. And then, finally, there were the serious psychiatric sequelae. Fourteen of the twenty-eight patients required re-detention in a psychiatric hospital, one on seven occasions, including five admissions occasioned by suicide attempts.
Despite the above reported adverse events, Helen Mayberg’s assessment of DBS is that it appears to be “generally safe and well tolerated.” Although the DBS studies failed to show any real efficacy with treatment-resistant depression, Mayburg concluded most participants had “a robust and sustained antidepressant response.” Andrew Scull pointed out many of the authors were indebted financially to the medical device manufacturer and that Mayburg owned patents covering the devices in question. Further, he said Mayberg et al “cherry-picked data” for their study. Positive outcomes only appeared when researchers self-assessed the results of their interventions. “Ambitious clinicians let loose in such a situation can easily be carried away by their enthusiasms, and the restraints on experimentation, while stronger than they once were, remain inadequate.”
Scull had a different take on the first-in-the-U.S. clinical trial for treatment resistant opioid use disorder described in Part 1. He pointed out the false impression given by saying the DBS device functioned much like a heart pacemaker. The human brain is not a simple pump. And despite all the progress of neuroscience over the past fifty years, our understanding of it is still primitive. “The idea of a ‘brain pacemaker’ is so ludicrous on its face as to disqualify anyone who uses it.” The analogy appears to rely on the now generally disparaged “chemical imbalance” theory of depression, as evident in the following statement:
By sending a pulsed current through the electrodes, doctors believe they can regulate an imbalance in Buckhalter’s reward circuitry.
Ali Rezai, the doctor leading the clinical trial, readily acknowledged they don’t fully understand how this works. However, they hope that by modulating the rewards circuit, which relies on the chemical messenger dopamine, “you’re getting better control, so you’re not craving dopamine as much.” This suggests DBS for opioid misuse is more explorative and experimental than it is a promising type of treatment. Scull commented: “The prattle about dopamine that he proceeds to utter as a substitute for the scientific evidence we do not possess is an embarrassment — just speculation plucked out of thin air.”
In The Science of Addiction, Carleton Erickson said scientists once held that dopamine was the “pleasure transmitter.” He said this is a simplistic explanation of severe SUD—substance use disorder. Addiction is more than seeking of pleasure or avoiding pain or withdrawal. In addition to the nucleus accumbens (part of the brain’s pleasure or reward system), recent neurobiological findings and theories extend to areas of the brain the modulate meaning and emotional and cognitive memory. “Some drugs appear to be capable of affecting these adjunctive brain areas to transition drugs from pure pleasure to habitual use to severe SUD, where pleasure or pain is no longer important in maintaining drug-taking behavior.”
Reflecting on the continuing pursuit of brain stimulation with transcranial magnetic stimulation (TMS), transcranial direct current stimulation (tDCS), repetitive transcranial magnetic stimulation (rTMS) and invasive, deep brain stimulation (DBS), I am reminded of a two-hundred-year-old novel that Mary Shelly anonymously published on January 1, 1818, The Modern Prometheus; or Frankenstein. Shelley told her story within a literary technique known as a frame narrative—a story within a story. In the frame story, Captain Robert Walton and his crew set out to explore the North Pole sometime in the 18th century in order to expand scientific knowledge and hopefully achieve fame. They glimpsed a dog sled driven by a gigantic figure, but it disappeared into the snow. A few hours later, they found a frozen and emaciated Victor Frankenstein, who had been in pursuit of the gigantic creature.
Victor recognizes the same obsession in Walton that drove him to modern laboratory experimentation and creation of the “fiend.” As a warning to Walton, he then recounts the story of his life’s miseries that were a consequence of his obsession. With his last words, he told Walton to seek “happiness in tranquility and avoid ambition.” Let us hope that the pursuers of brain stimulation, particularly DBS, heed that counsel. Experimenting on humans by implanting electrodes into their brain to “treat” their depression or addiction seems like a similar pursuit of ambition over first doing no harm. Hopefully Derek Hutchinson’s life story and its miseries will be heard and give pause to some of these researchers.
Originally posted on February 4, 2020