Rebranding Problems With Abilify
Abilify had a profitable and expansive run for Otsuka Pharmaceuticals since it was approved by the FDA for the treatment of schizophrenia in 2002. The FDA then expanded its treatment use to include bipolar disorder in 2005, irritability associated with autistic disorder in 2009 and Tourette’s disorder in 2014. It was also approved by the FDA as an add-on with antidepressants for treatment-resistant depression in 2007. Abilify is used off-label to treat insomnia, delusional disorders and anxiety. In 2013 this enlarged treatment market brought in $7.8 billion in global sales for Otsuka. FiercePharma noted the end of Abilify’s patent exclusivity in April of 2015 left a big revenue hole for Otsuka and Bristol-Myers Squibb, who marketed and promoted it in the U.S. But instead of quietly surrendering to the generic market, Abilify (aripiprazole) was rebranded into three new drug applications with the FDA: Abilify Maintena, Abilify MyCite and Aristada.
Abilify Maintena was approved as an extended-release once-monthly depot shot of aripiprazole for the treatment of schizophrenia in March of 2013. Otsuka said it was a new treatment option to address the need for relapse prevention in patients with schizophrenia. It was the first dopamine D2 partial agonist approved as a once-monthly injection. In July of 2017 its approved use was extended to include maintenance monotherapy treatment of Bipolar I Disorder in adults.
Abilify MyCite has an ingestible sensor embedded in aripiprazole tablets that records that the medication was taken. Approved by the FDA in November of 2017, it is the first drug in the U.S. with a digital tracking system. This was a second try for Otsuka, as the FDA initially failed to approve Abilify MyCite in April of 2016, saying it needs more information about the product’s use, and further human factor investigations. “The goal of human factors testing is to evaluate use-related risks and confirm that users can use the device safely and effectively.”
The pill’s sensor sends a message to a wearable patch that in turn transmits the information to a mobile application that allows the patient to track the ingestion of the medication on their smart phone. Patients can give their caregivers and doctors permission to access the information through a web-based portal. Mitchell Mathis, the director of the Division of Psychiatry Products in the FDA’s Center for Drug Evaluation and Research, said: “Being able to track ingestion of medications prescribed for mental illness may be useful to some patients.” Actually, the digital tracking capability of Abilify MyCite is a selling point for psychiatrists concerned with patient adherence. Getting people who are prescribed antipsychotics to stay on their drugs can be a challenge. See “Doublespeak With Abilify MyCite” for more information on this.
Aristada (aripiprazole lauroxil) was approved by the FDA as an extended release injectable form of aripiprazole to treat adults with schizophrenia on October 5, 2015. It is a prodrug, meaning it is a biologically inactive compound that is metabolized into a pharmacologically active drug within the body. Unlike Abilify MyCite and Abilify Maintena, its parent company is Alkermes, not Otsuka. Similar to the price of another Alkermes drug, Vivitrol, Aristada costs about $1,500 per month.
According to ProPublica, Alkermes has successfully marketed Vivitrol to the criminal-justice system, emphasizing its use in drug courts. The Atlantic wrote how the relationship between drug companies and the criminal-justice system has expanded in “Marketing Psychiatric Drugs to Jailers and Judges,” as drug companies realized the potential market behind bars. Free samples are distributed to detention facilities; jail and prison doctors attend free luncheons to learn about medications. Payments are made to doctors and criminal-justice employees, such as sheriffs and drug-court judges, to promote certain medications.
Alkermes and other drug companies have marketed not only to jailers but to judges as well. Earlier this year, at a conference for drug- and mental-health-court professionals in Maryland, Alkermes sponsored a closed-door promotional session about using long-acting shots in a court setting. Featured at the session was Richard Jackson, a former psychiatrist at the Women’s Huron Valley Correctional Facility in Ypsilanti, Michigan, and Ernie Glenn, a magistrate in Bexar County, Texas, who had helped defendants in his court get access to long-acting antipsychotic shots. While Glenn had received no payments from Alkermes, the company had paid Jackson more than $250,000 between 2015 and 2018 for speeches, travel and lodging, and meals, according to the Centers for Medicare and Medicaid Services’s open payments database. (Jackson also received $252,608 in payments from Otsuka from 2015 to 2018, and said he has continued receiving payments from drug companies in 2019; it wasn’t immediately clear whether Alkermes was one of them.) The conference program, as in the conference in Nashville, directed people to learn about Aristada at Alkermes’s exhibit booth.
The director of the ACLU’s National Prison Project, David Fathi, expressed concern that the marketing has been aimed at judges and prison officials instead of the incarcerated people themselves. A ProPublica analysis found that doctors who accepted money from pharmaceutical companies were more likely to prescribe those companies’ medications. Incarcerated patients may not feel they have a real ability to choose. In cases where patients choose to take a psychiatric drug, it may be a choice made under duress. “If you know you can be forcibly medicated, can you really make a free and noncoercive choice about medication?”
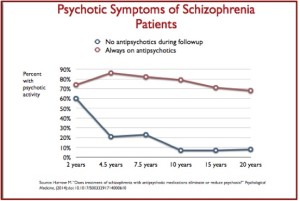
The makers of drugs like Abilify Maintena and Aristada are banking on long-acting injections as the future of schizophrenia treatment. A 2015 study found that patients who were given injections of long-acting risperidone were more likely to comply with treatment and led to better long-term outcomes. The lead author of the study, Kenneth Subotnik, said, “We know that not taking antipsychotic medication is the single greatest modifiable risk factor for psychotic symptoms returning.” Yet as Max Blau noted in The Atlantic, there is a possibility the side effects will last longer than with the pill form. The most common side effect with Aristada is akathisia (a feeling of inner restlessness and inability to stay still). Other side effects include: Neuroleptic Malignant Syndrome, Tardive Dyskinesia, Diabetes, weight gain and seizures.
In 2016 the FDA published a Safety Communication about impulse-control problems associated with aripiprazole (Abilify, Abilify Maintena and Aristada). They warned of reports of compulsive, uncontrollable urges to gamble, binge eat, shop, and have sex with the use of aripiprazole. The impulse-control problems were rare, but may result in harm to the patient or others. “These uncontrollable urges were reported to have stopped when the medicine was discontinued or the dose was reduced.”
Although pathological gambling is listed as a reported side effect in the current aripiprazole drug labels, this description does not entirely reflect the nature of the impulse-control risk that we identified. In addition, we have become aware of other compulsive behaviors associated with aripiprazole, such as compulsive eating, shopping, and sexual actions. These compulsive behaviors can affect anyone who is taking the medicine. As a result, we are adding new warnings about all of these compulsive behaviors to the drug labels and the patient Medication Guides for all aripiprazole products.
A search of the FDA Adverse Event Reporting System (FAERS) database and the medical literature identified 184 case reports with an association between arpiprazole use and impulse-control problems. Pathological gambling was the most common with 164 cases, “but other compulsive behaviors including compulsive eating, spending or shopping, and sexual behaviors were also reported.” None of the patients had a history of pathological gambling, compulsive sexual behavior, binge eating or compulsive shopping before starting arpiprazole treatment. None of the patients had concurrent substance abuse disorder or symptoms of mania at the time of developing the impulse-control problems.
Despite the problems with Abilify, Otsuka and Alkermes have repackaged it with digital tracking technology and a slow-release, depot injection and then promoted these products as improving treatment adherence, reducing the risk of overdose and improving relapse prevention and reducing rehospitalization rates.
Pharmaceutical companies seem to disregard the problems with antipsychotics like Abilify while they pursue their potential profits. Bristol-Myers Squibb paid $515 million to settle charges it illegally marketed Abilify for children and the elderly. See “Broken Promises with Ability” for more information on this issue. Persistence in addressing problems with nonadherence seems to ignore the larger issue of adverse side effects antipsychotics. See “Abilify in Denial,” “Pick Your Poison: Diabetes and Psych Meds,” “Downward Spiral of Antipsychotics,” “Pros and Cons of Antipsychotics” and “Biomedical Big Brother” for more on concerns with adherence and adverse side effects with Abilify and other antipsychotics. The rebranding Abilify as Abilify Maintena, Abilify MyCite and Aristada does not appear to address the concerns with aripiprazole and other antipsychotics.