Protestants Without Sola Fide, Part 2

© kehli | 123rf.com- monument of Martin Luther on the market place in front of the townhall, Wittenberg, Germany.
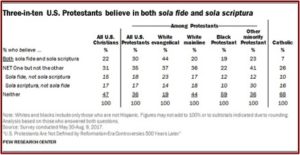
Some of the reported findings of the Pew Research Center’s “U.S. Protestants Are Not Defined by Reformation Era Controversies” have left more than one person scratching their heads. Surprisingly, only 27% of Protestants correctly said that Protestants alone traditionally teach that salvation comes through faith alone; 44% of Protestants said that both Catholics and Protestants hold this doctrinal belief. Confusingly, the survey also found that 24% of self-identified Protestants said they were not familiar with the term “Protestant.” What does it mean when 24% of Protestants aren’t familiar with the term “Protestants”?
In Part One of this article, I looked at the confusion with how Pew defined one of the fundamental doctrinal differences between Protestants and Catholics, sola fide: justification by faith alone. The Pew survey linked sola fide with salvation and not justification within the questionnaire when it said: “faith in God alone is needed to get into heaven.” This Pew definition was not the classic Protestant understanding of sola fide. Nevertheless, only 46% of self-identified Protestants said they thought “Faith in God is the only thing that gets people into heaven,” 52% said: “Both good deeds and faith in God are necessary to get into heaven”—a Roman Catholic sense of salvation.
The Pew study does seem to suggest that: “U.S. Protestants are not united about – and in some cases, are not even aware of – some of the controversies that were central to the historical schism between Protestantism and Catholicism.” Most American Protestants (57%) believe the two Christian traditions are more alike than different. And as noted above, 52% of Protestants believe that faith and works are necessary for salvation. This is a dramatic shift from the position of the Reformation.
In his article on justification in the New Dictionary of Theology, N.T. Wright said popular Protestantism has suppressed the distinction between justification and regeneration, while Roman Catholics have continued to be influenced by Augustine, “who saw it as God’s action in making people righteous, through pouring into their hearts love towards himself.” The emphasis on the change brought about by God’s action has continued into modern Roman Catholic theology, with the consequence that the reference of the word has been broadened significantly “to include far more than Paul (or the Reformers) intended.” So it seems worthwhile to review here the essential Protestant doctrines, noting where they are distinct from Roman Catholicism.
Writing for Themelios, Scott Mantesch said in “Is the Reformation Over?” that Protestant Christians often summarize their primary doctrinal commitments with the five “solas”: sola Scriptura, sola gratia, sola fide, solus Christus, and soli Deo Gloria. He noted that Calvin wrote the five solas should not be treated as discrete or independent doctrines. “They cohere with, inform, and require other important biblical truths.” For example, there is a theological inconsistency in affirming the doctrine of justification by faith alone, while remaining committed to the sacrament of penance. “Calvin recognized that whatever authority the Catholic Church ascribed to Scripture in theory, Rome undermined Scripture’s authority in practice by commanding the exclusive right of interpreting the biblical text.”
P. Chase Sears said in his article on “New Testament Theology” for the Lexham Bible Dictionary that the Reformers insisted that the church return to sola Scriptura—Scripture alone as the authoritative source for theology. They emphasized the grammatical-historical method of interpretation in order “to grasp the overall structure of the biblical understanding of God and his relations with mankind.” Martin Luther saw Jesus Christ as the heart of Scripture, but he wrestled with the problem of unity in diversity within Scripture. In order to resolve this difficulty, he distinguished between law and gospel, “with the doctrine of justification by faith as his hermeneutical key to piecing the entire Bible together.”
Mantesch said the following on how the Reformer’s understanding of the doctrine of justification by faith distinguished Protestant and Roman Catholic theology from each other:
For the Protestant reformers, justification was a first-order doctrinal concern. Not so with many contemporary Catholics. The most recent edition of the Catholic Catechism gives only brief attention to the doctrine of justification. Clearly, sacramental grace, not justification, occupies the central position in Catholic conceptions of salvation. American Cardinal Avery Dulles admits as much: “Justification is rarely discussed at length except in polemics against, or dialogue with, Protestants.” It is noteworthy that the official Catholic formulations of the doctrine of justification found in the “Catholic Catechism” and the “Joint Declaration” make no mention of the positive forensic character of justification—that sinners are acquitted before God on account of the imputed righteousness of Christ. Moreover, both of these documents describe justification as including divine pardon and the process of renewal of the inner person. The “Catholic Catechism,” for example, reaffirms the definition of justification formulated at Trent in 1547: “Justification is not only the remission of sins, but also the sanctification and renewal of the interior man.” [See Chapter VII of the Council of Trent]
Mantesch then asked how Protestants should respond when contemporary Roman Catholic churchmen affirm one or several of the solas. In the Joint Declaration of 1999 Roman Catholic officials approved “by grace alone” and “by faith alone.” Responding to his own question, he said we should be grateful that Catholics are willing to affirm these central biblical truths, while remaining cautious and realistic.
The Gospel Coalition now has a series of free online courses available, one of which is on The Five Solas. In “Remembering the Reformation by Reflecting on its Solas,” Stephen Wellum said: “The solas remind us about the God-centered nature of Christianity and how human beings, as important as we are as image-bearers, are completely dependent upon God’s sovereign initiative to create, reveal, rule, and redeem.” Each of the five solas is addressed and there is a wealth of information (written and video) on each doctrine.
Similarly, in his lecture on “Sola Fide: Lady Jane Grey & the Rediscovery of Justification by Faith,” Steve Nichols said: “Salvation from start to finish is the work of God for his glory.” The doctrine of justification reminds us that we don’t have to do anything for our salvation. Indeed, we cannot. “Christ has done it for us.” Nichols went on to suggest that Martin Luther used two words to describe the doctrine of justification: alien and immediate. By alien he meant justification is outside of us. “The doctrine of justification reminds us that it is nothing that we muster.” By immediate, Luther meant “without a mediator.” Where the medieval Catholic church saw salvation between Christ and sinful humanity mediated by the church and its sacraments, for Luther there was no mediator between humanity and Christ. The video for the Nichols lecture is under The Gospel Coalition material on Sola Fide. A link there will take you to Ligioner Ministries, where there are additional links to the lectures Dr. Nichols gave on the five solas.
In its attempt to assess differences between Protestants and Roman Catholics on one of the classic Reformation solas, The Pew Research Center study treated sola fide as a discrete and independent doctrine from the remaining four, something John Calvin said should not be done. Together the five solas, “cohere with, inform, and require other important biblical truths.” Additionally, by removing the term justification from its definition of sola fide and asking participants to choose whether “faith in God” or “good deeds and faith in God” were necessary for salvation, Pew effectively neutered what was a first-order doctrinal concern for Protestants—at the time of the Reformation and today.
Thomas Schreiner in his lecture on Sola Fide for the 2015 Theology Conference at Southern Baptist Theological Seminary, said it was right to say faith alone saves us “since imperfect works don’t pass muster to make us right with God.” Good works are necessary for eternal life, but they can’t be the basis for our right-standing with God since He demands perfection. “Good works are a fruit of faith and a result of the Spirit’s work.” As Shreiner and others have said: “We are justified by faith alone and yet our faith is never alone.” In conclusion, I think it’s worth repeating another statement Schreiner made in his lecture:
When we speak of justification by faith alone, we aren’t saying that our faith justifies us. We see here how the five solas are closely linked together, for righteousness is by faith alone because our righteousness is in Christ alone as the crucified and risen one. And if our righteousness is by faith alone and in Christ alone, then it is by grace alone since our works don’t constitute our righteousness. And our righteousness is also to the glory of God alone since he is the one who has accomplished our salvation. Justification by faith alone doesn’t call attention to our faith but to Christ as the redeemer, reconciler, and Savior. [As an aside in the video, Schreiner noted he didn’t mention sola Scriptura here. “But everything I said supported that.”]